What is the Heisenberg Uncertainty Principle? - Chad Orzel
TLDRThe Heisenberg Uncertainty Principle, a cornerstone of quantum mechanics, asserts that it's impossible to know both the exact position and speed of an object simultaneously. This principle arises from quantum objects behaving as both particles and waves, with particles having a definite position and waves being spread out. The more precisely one property is measured, the less certain the other becomes, reflecting a fundamental limit on what can be known about the properties of objects, inherent to the universe's structure.
Takeaways
- 🌌 The Heisenberg Uncertainty Principle is a fundamental concept in quantum physics that has also permeated into general pop culture.
- 📏 It asserts that it is impossible to know both the exact position and exact speed (momentum) of an object simultaneously.
- 🔍 The principle is often misconceived as a limitation of measurement, but it fundamentally arises from the wave-particle duality of quantum objects.
- 🌊 In quantum mechanics, objects exhibit properties of both particles and waves, which means they can't have a definite position and momentum at the same time.
- 📊 Particles are represented by a spike in a probability graph, indicating a 100% chance of being at a specific position and zero elsewhere.
- 🌀 Waves, conversely, are spread out, with features like wavelength identifiable but no single position, indicating a probability of being in multiple places.
- 🔗 The wavelength of an object is crucial as it is related to its momentum, which is the product of mass and velocity.
- 🏀 Everyday objects like a baseball don't exhibit their wave nature due to their extremely short wavelengths, which are imperceptible.
- ⚛️ Smaller entities like atoms or electrons, however, can have wavelengths significant enough to be measured in experiments.
- 🌐 To reconcile the particle and wave nature, a wave packet is created by combining waves of different wavelengths, resulting in a region of high probability for both position and momentum but with inherent uncertainty.
- 🔄 The Heisenberg Uncertainty Principle dictates a trade-off: reducing uncertainty in position increases momentum uncertainty and vice versa.
- 📝 This principle, first articulated by Werner Heisenberg in 1927, is not just a measurement limitation but a fundamental property of the universe, reflecting the intrinsic limits of what an object can be known to possess.
Q & A
What is the Heisenberg Uncertainty Principle?
-The Heisenberg Uncertainty Principle states that it is impossible to simultaneously know the exact position and the exact speed (momentum) of an object at the same time. It is a fundamental concept in quantum mechanics.
Why has the Heisenberg Uncertainty Principle become popular outside of physics?
-The Heisenberg Uncertainty Principle has become popular in general pop culture because it is often used metaphorically to represent the inherent uncertainty in various fields, such as literary criticism and sports commentary.
What is the common misconception about the origin of the Uncertainty Principle?
-A common misconception is that the Uncertainty Principle is due to the act of measurement affecting the object being measured. However, the true origin is more profound, stemming from the dual particle-wave nature of all objects in the universe.
How does the dual nature of particles and waves in quantum mechanics relate to the Uncertainty Principle?
-The dual nature of particles and waves is fundamental to the Uncertainty Principle because particles have a definite position but no wavelength, while waves have a wavelength but no definite position. Combining these properties results in an inherent uncertainty in both position and momentum.
What is the significance of wavelength in quantum physics?
-In quantum physics, wavelength is significant because it is related to an object's momentum (mass times velocity). A fast-moving or heavy object has a short wavelength, which is indicative of its momentum.
Why don't we notice the wave nature of everyday objects like a baseball?
-The wave nature of everyday objects like a baseball is not noticeable because their wavelengths are extremely small, to the point of being undetectable, due to their mass and speed.
How can wavelengths be measured in physics experiments?
-Wavelengths can be measured in physics experiments for small objects like atoms or electrons, which can have wavelengths large enough to be detected and measured.
What is a wave packet in the context of quantum mechanics?
-A wave packet in quantum mechanics is a region where waves of different wavelengths combine to create a localized packet with a clear wavelength, representing an object with both wave and particle properties.
How does creating a wave packet affect the certainty of an object's position and momentum?
-Creating a wave packet involves combining waves with different wavelengths, which results in an object having a range of possible positions and momenta, thus increasing the uncertainty in both properties.
What is the relationship between position uncertainty and momentum uncertainty in the Heisenberg Uncertainty Principle?
-The relationship is that if you reduce the position uncertainty by making a smaller wave packet, you increase the momentum uncertainty because you are adding more waves. Conversely, to know the momentum better, you need a bigger wave packet, which increases position uncertainty.
Who first stated the Heisenberg Uncertainty Principle, and when was it stated?
-The Heisenberg Uncertainty Principle was first stated by German physicist Werner Heisenberg in 1927.
What does the Heisenberg Uncertainty Principle imply about the fundamental structure of the universe?
-The Heisenberg Uncertainty Principle implies that the universe's fundamental structure inherently limits the properties an object can have, such as exact position and momentum, and is not just a practical limit on measurement.
Outlines
🌌 The Heisenberg Uncertainty Principle
This paragraph introduces the Heisenberg Uncertainty Principle, a fundamental concept in quantum physics that has permeated popular culture. It explains that the principle states the impossibility of knowing both the exact position and exact speed of an object simultaneously. The explanation delves into the quantum mechanical behavior of particles and waves, emphasizing that particles are localized in space, while waves are spread out. The principle's origin is attributed to the dual nature of quantum objects behaving as both particles and waves, with their wavelengths related to their momentum. The paragraph also discusses how everyday objects' wave nature is undetectable due to their minuscule wavelengths, whereas atomic or subatomic particles have measurable wavelengths. It concludes by explaining the creation of a wave packet that embodies both particle and wave characteristics, inevitably introducing uncertainty in both position and momentum, which is the essence of the Heisenberg Uncertainty Principle.
Mindmap
Keywords
💡Heisenberg Uncertainty Principle
💡Quantum Physics
💡Measurement
💡Particle
💡Wave
💡Wavelength
💡Momentum
💡Wave Packet
💡Position Uncertainty
💡Momentum Uncertainty
💡Fundamental Structure
Highlights
The Heisenberg Uncertainty Principle is a fundamental concept in quantum physics that has permeated into general pop culture.
It states that one cannot know both the exact position and exact speed of an object simultaneously.
The principle is often misunderstood as a result of measurement, but it originates from the wave-particle duality of quantum objects.
In quantum mechanics, the exact position and speed of an object are not well-defined.
Particles are defined by existing in a single place at any given time, represented by a spike in a probability graph.
Waves are disturbances spread out in space, unlike particles, and do not have a single position.
Wavelength is a key concept in quantum physics, relating to an object's momentum.
Fast-moving or heavy objects have short wavelengths, making their wave nature less noticeable.
Small objects like atoms or electrons can exhibit wavelengths measurable in experiments.
A pure wave can be measured for wavelength and momentum but lacks a specific position.
A particle can have a known position but lacks a wavelength, and thus its momentum is unknown.
Combining waves with different wavelengths allows for the creation of a wave packet with both wave and particle properties.
Creating a wave packet involves a trade-off between certainty in position and momentum.
The Heisenberg Uncertainty Principle was first stated by Werner Heisenberg in 1927.
The principle is not about measurement limitations but an inherent property of the universe's structure.
The uncertainty is not due to measurement quality but a fundamental aspect of quantum objects' behavior.
The Uncertainty Principle sets a fundamental limit on the properties an object can possess.
Transcripts
Browse More Related Video

The Heisenberg Uncertainty Principle Part 1: Position/Momentum and Schrödinger's Cat

What is the Heisenberg Uncertainty Principle? A wave packet approach

Lecture 10 | The Theoretical Minimum

Neil deGrasse Tyson Explains The Weirdness of Quantum Physics
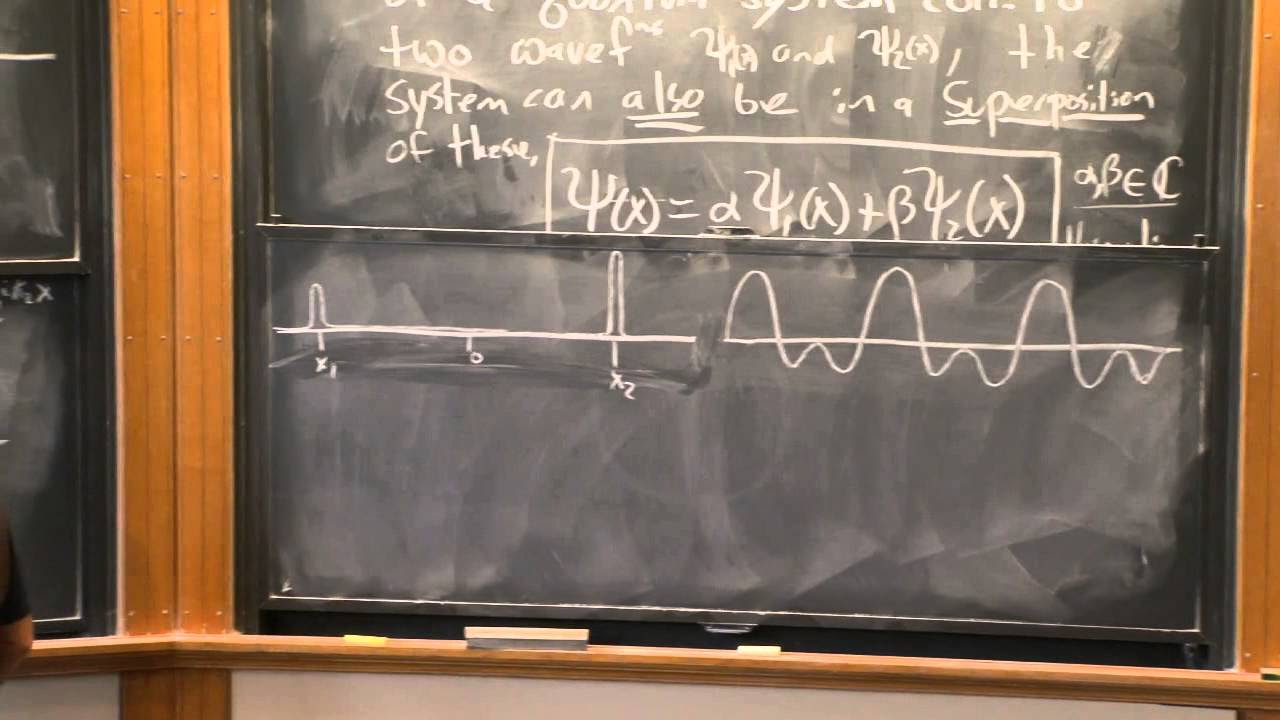
Lecture 3: The Wave Function

Lec-11 I Introduction to quantum chemistry I Applied Chemistry I Chemical Engineering
5.0 / 5 (0 votes)
Thanks for rating: