ALEKS: Deriving Kb from Ka
TLDRThis video tutorial explains how to solve the Aleks problem involving the relationship between the equilibrium constants ka and kb, where ka * kb = Kw (1 x 10^-14). It demonstrates how to calculate ka from kb and vice versa, and also covers how to determine the formulas for conjugate acids and bases from given molecular formulas.
Takeaways
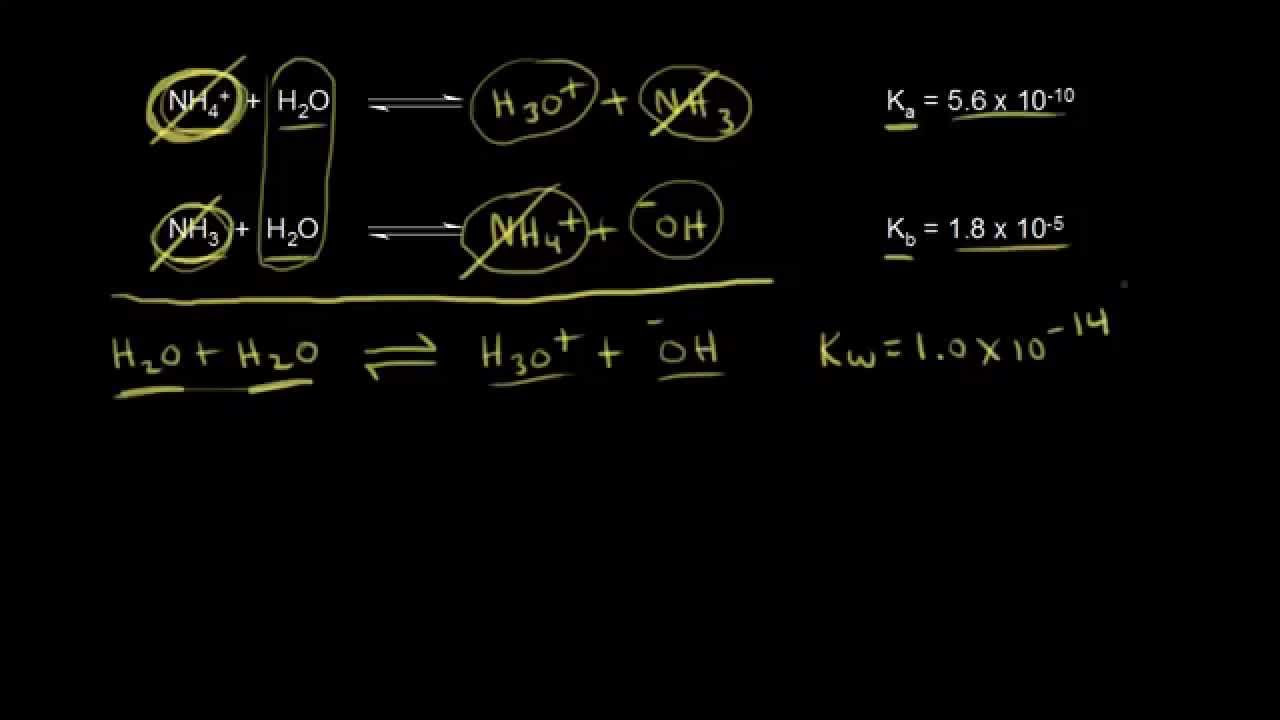
- 🔍 The video explains how to solve a chemistry problem involving the relationship between the acid dissociation constant (Ka) and the base dissociation constant (Kb), where Ka * Kb = Kw, with Kw being a constant value of 1 × 10^-14.
- 📚 To find Ka when given Kb, divide Kw by Kb, and vice versa, to find Kb when given Ka.
- 📝 The script provides three sets of data, where the first set involves calculating Ka from a given Kb value.
- 🧮 For the first set, the calculation of Ka is demonstrated using the formula Ka = Kw / Kb, with Kw = 1 × 10^-14 and a given Kb of 1.1 × 10^-8, resulting in Ka = 9.1 × 10^-7.
- ❗ A common mistake students make is incorrectly setting up the problem by flipping the numbers, such as dividing Kb by Kw instead of the correct approach.
- 🔄 The third set of data involves a similar calculation but in reverse, calculating Kb from a given Ka value.
- 🔢 The second row of the problem is the opposite of the first, where Ka is given and Kb needs to be calculated using the formula Kb = Kw / Ka.
- 🤔 The script mentions that some students have difficulty with calculations involving very small or large numbers, like 5.6 × 10^-10.
- 🌐 The video also covers writing the formulas for the conjugate acid or base for given molecules, based on whether the molecule is an acid or a base.
- 💧 For acids, which are H+ donors, the conjugate base is formed by losing an H+ and possibly adjusting the charge.
- ➕ For bases, which are H+ acceptors, the conjugate acid is formed by gaining an H+ and possibly adjusting the charge, typically adding the H+ at the front of the formula.
- 📝 The script emphasizes the importance of correctly identifying and writing the formulas for the conjugate acid or base, especially noting that the placement of H+ and the adjustment of charge are crucial.
Q & A
What is the relationship between Ka and Kb in the context of the video?
-The relationship between Ka and Kb is given by the equation Ka * Kb = Kw, where Kw is the ion product of water, which is a constant value of 1 × 10^-14.
How can you calculate Ka if you know Kb and vice versa?
-You can calculate Ka if you know Kb by dividing Kw by Kb, and similarly, you can find Kb by dividing Kw by Ka.
What is the value of Kw used in the video?
-The value of Kw used in the video is 1 × 10^-14.
How is the first set of data in the video used to find Ka?
-In the first set of data, a Kb value is given, and to find the corresponding Ka, you divide Kw by the provided Kb value.
What is the calculated Ka value for the first set of data in the video?
-The calculated Ka value for the first set of data is 9.1 × 10^-7.
What is the common mistake students make when solving for Ka or Kb in this problem?
-The common mistake students make is flip-flopping the numbers, such as dividing Kb by Kw instead of Kw by Kb.
What is the approach when you are given Ka and asked to calculate Kb?
-When given Ka and asked to calculate Kb, you divide Kw by Ka.
How does the video explain the process of finding the conjugate acid or base of a molecule?
-The video explains that if a molecule is an acid (H+ donor), its conjugate base is formed by losing an H+. Conversely, if a molecule is a base (H+ acceptor), its conjugate acid is formed by gaining an H+.
What is the formula for the conjugate acid of CH3CO2- as mentioned in the video?
-The formula for the conjugate acid of CH3CO2- is CH3CO2H, which is formed by adding an H+ to the base.
How does the video describe the process of writing the formula for the conjugate base of an acid?
-The video describes that to write the formula for the conjugate base of an acid, you add an H+ to the front of the formula and adjust the charge to be neutral.
What is the charge of the conjugate acid formed when a base gains an H+?
-The charge of the conjugate acid formed when a base gains an H+ is typically a positive charge, as it is formed by adding an H+ to the base.
Outlines
🔍 Deriving Ka from Kb in Chemistry
This paragraph explains how to solve a chemistry problem involving the relationship between the acid dissociation constant (Ka) and the base dissociation constant (Kb). The key equation is presented as Ka * Kb = Kw, where Kw is the ion product of water, a constant value of 1 × 10^-14. The video demonstrates how to calculate Ka when given Kb, and vice versa, using the provided equation. The example given involves dividing Kw by the known Kb value to find Ka, resulting in a significant figure calculation. The speaker also cautions against common mistakes, such as incorrectly setting up the equation, which could lead to incorrect results.
📚 Writing Formulas for Conjugate Acids and Bases
The second paragraph delves into the process of determining the formulas for conjugate acids and bases given the initial molecule. It explains that acids are H+ donors and bases are H+ acceptors, and how to adjust the chemical formulas accordingly. For acids, one H+ is removed and the charge is adjusted, while for bases, an H+ is added along with a positive charge to maintain neutrality. The paragraph provides specific examples, such as transforming CH3CO2 (a base) into its conjugate acid by removing an H+ and adjusting the charge, and vice versa for HClO, which is turned into its conjugate acid by adding an H+ and a positive charge. The importance of correctly placing the hydrogen in the molecular formula, especially when nitrogen is involved, is emphasized, as is the need to adjust the overall charge of the molecule.
Mindmap
Keywords
💡Aleks
💡Deriving
💡Ka
💡Kb
💡Kw
💡Equilibrium Constant
💡Conjugate Acid
💡Conjugate Base
💡Significant Figures
💡Mistake
💡Charge
Highlights
The video demonstrates solving the Aleks problem involving the relationship between acid dissociation constants, ka and kb.
The fundamental equation is ka * kb = kw, where kw is a constant (1 * 10^-14).
If the value of kb is known, ka can be calculated and vice versa.
The video provides three sets of data for deriving ka from kb.
In the first set, the value of kb is given, and the task is to calculate the corresponding ka.
The formula to solve for ka is ka = kw / kb.
The provided value of kw is 1 * 10^-14, and a sample kb value is 1.1 * 10^-8.
Calculating ka results in 9.1 * 10^-7, rounded to two significant figures.
The third set of data involves a similar approach to calculate ka from a given kb.
The video warns about the common mistake of flip-flopping numbers in calculations.
The second row of the problem involves calculating kb when given the value of ka.
The formula for calculating kb is kb = kw / ka.
An example calculation uses a ka value of 1.8 * 10^-5.
The video notes potential calculator issues with very small or large numbers.
The problem also includes writing formulas for conjugate acids and bases.
Acid molecules are identified as H+ donors, and their conjugate bases are formed by losing H+.
Base molecules are H+ acceptors, and their conjugate acids are formed by gaining H+.
The video provides specific examples of how to derive the formulas for conjugate acids and bases.
For acids, the conjugate base formula involves removing a hydrogen and adjusting the charge.
For bases, the conjugate acid formula involves adding a hydrogen and a positive charge.
The video emphasizes the importance of correct placement of hydrogen atoms in the molecular formulas.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: