ALEKS: Understanding that no reaction goes to 100% completion
TLDRThis video explains how to solve a chemistry problem involving a reversible reaction where no reaction goes to 100% completion. It introduces the concept of millimoles and uses a chemical equation to demonstrate how to determine the amounts of reactants and products at equilibrium, highlighting that reactions do not proceed to complete conversion.
Takeaways
- 🔬 In the video, the presenter explains the ALEKS problem titled 'Understanding That No Reaction Goes to 100% Completion,' emphasizing how chemical reactions never completely reach 100% conversion.
- 📏 The unit millimole (mmol) is introduced, explained as 10^-3 of a mole, similar to metric units like milliliter or millimeter, useful for representing small quantities in chemical calculations.
- 🧪 In the first scenario, 160 millimoles of NOCl are added to an empty flask. The task is to determine the amount of NO present at equilibrium, highlighting that the reaction does not go to full completion.
- ⚖️ Because the reaction is in equilibrium, some NOCl will react, producing NO and Cl, but not all of it. The concept of equilibrium means only a portion of reactants will convert into products.
- ❌ The option of having 'none' of NO is incorrect because some NOCl will react, resulting in more than zero NO being present.
- 📉 Not all 160 millimoles of NOCl will react; hence, there will be less than 160 millimoles of NOCl remaining, reinforcing the concept that reactions do not reach complete conversion.
- ⚠️ More than 160 millimoles of NO is impossible since NOCl and NO react in a 1:1 ratio, making 160 millimoles the maximum achievable amount under ideal conditions.
- 🔄 In the second scenario, 140 millimoles of NO and 140 millimoles of Cl are placed in an empty flask, and the problem is to calculate the amount of NOCl formed.
- ➗ Some of the 140 millimoles of NO and Cl will react to form NOCl, but not all of it. Thus, there will be more than zero but less than 140 millimoles of NOCl.
- ♻️ The reaction not going completely from NO and Cl to NOCl emphasizes the presence of equilibrium, indicating that some reactants remain unreacted.
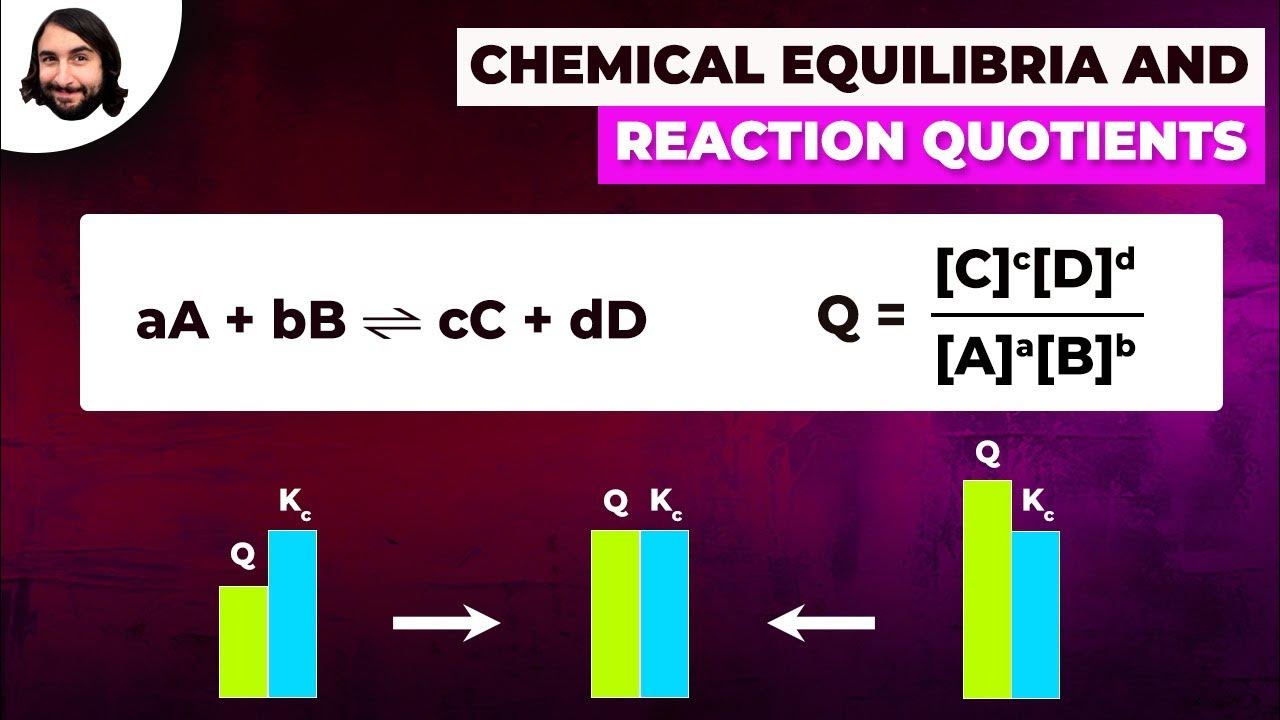
- ⚖️ The key takeaway is that chemical reactions often reach a state of dynamic equilibrium, where the rate of the forward reaction equals the rate of the reverse reaction, preventing full conversion.
Q & A
What is the main topic of the video?
-The video discusses how to solve a chemistry problem involving understanding that no chemical reaction goes to 100% completion.
What is the significance of the term 'millimole' in the context of this problem?
-A millimole (mmol) is a unit of measurement used to describe a small quantity of a substance, equivalent to 10 to the minus third of a mole. It is useful when dealing with very small amounts of reactants or products in a chemical reaction.
What is the chemical reaction being discussed in the video?
-The chemical reaction discussed is the equilibrium between NOCl and NO and Cl, represented by the equation NOCl ⇌ NO + Cl.
Why is it incorrect to say that all 160 millimoles of NOCl will react in the first scenario?
-It is incorrect because the reaction is at equilibrium, meaning that not all of the NOCl will react. Some will be converted into NO and Cl, but some will remain as NOCl.
What is the incorrect assumption made by choosing the answer 'more than 160 millimoles' in the first scenario?
-The incorrect assumption is that the reaction only proceeds in one direction (from NOCl to NO and Cl) without any back reaction, which violates the principle of equilibrium.
In the second scenario, why can't all 140 millimoles of NO and Cl react to form NOCl?
-Because the reaction is at equilibrium, not all of the NO and Cl will react to form NOCl. Some will remain as NO and Cl, and some will form NOCl.
What does it mean when the video script says 'the reaction is not going to go a hundred percent to completion'?
-This means that the reaction will not convert all reactants into products. Some reactants will remain unreacted at equilibrium.
Why is the answer 'exactly 140 millimoles' not appropriate for the second scenario?
-This answer would imply that the reaction goes only from NO and Cl to NOCl without any back reaction, which is not the case in an equilibrium reaction.
What is the law of conservation of mass, and how does it relate to the problem discussed in the video?
-The law of conservation of mass states that mass cannot be created or destroyed in a chemical reaction. In the context of the problem, it means that the total mass of reactants and products must be equal, and you cannot end up with more mass of NOCl than was initially present as NO and Cl.
What is the equilibrium arrow, and why is it used instead of a forward arrow in the video?
-The equilibrium arrow (⇌) is used to represent a reversible reaction at equilibrium, where the forward and reverse reactions occur at the same rate. It is used instead of a forward arrow to indicate that the reaction is not going to completion and that both reactants and products are present at equilibrium.
Outlines
🔍 Introduction to Equilibrium Reactions
This video covers solving the ALEKS problem focused on understanding that no reaction goes to 100% completion. The problem presents a chemical equation and requires answering two separate questions about it. It introduces the millimole (mmol), a unit used for very small quantities, where 1 mmol equals 10^-3 moles. The speaker rewrites the chemical reaction to have more space for working through the problem.
⚗️ First Scenario: Adding NOCl
In the first scenario, 160 millimoles of NOCl are added to an empty flask, meaning no products are initially present. The task is to determine the amount of NO at equilibrium. Since the reaction is at equilibrium, some of the NOCl will react, but not all. Therefore, there will be some amount of NO produced, but less than 160 millimoles. The answer cannot be zero, 160, or more than 160 millimoles due to the nature of equilibrium reactions and stoichiometry.
🔄 Second Scenario: Forming NOCl
The second scenario starts with 140 millimoles each of NO and Cl in an empty flask, and the goal is to determine the amount of NOCl formed. Some of the NO and Cl will react, producing NOCl, but not all 140 millimoles. Hence, the amount of NOCl will be more than zero but less than 140 millimoles. The options of none, exactly 140, or more than 140 millimoles are invalid due to the equilibrium condition and the law of conservation of mass.
Mindmap
Keywords
💡Aleks
💡No reaction goes to 100% completion
💡Chemical equation
💡Millimole (mmol)
💡Equilibrium
💡Flask
💡Reaction arrow
💡Products
💡Reactants
💡Conservation of mass
Highlights
The video explains how to solve an Aleks chemistry problem about reactions not going to 100% completion.
The problem involves a chemical equation with two separate, unrelated questions.
Introduces the millimole (mmol) unit, which is 10^-3 mole, useful for small quantities.
First scenario: 160 mmol of NOCl added to an empty flask, no products initially.
At equilibrium, some NOCl will react to form NO and Cl, but not all of it.
Equilibrium means there will be some, but less than 160 mmol of NOCl remaining.
The best answer choice is 'some, but less than 160' for the amount of NOCl left.
Second scenario starts with 140 mmol each of NO and Cl, and no initial NOCl.
The reaction will form some NOCl, but not all 140 mmol will react.
The amount of NOCl formed will be more than zero but less than 140 mmol.
The reaction cannot go 100% to completion, so NOCl will not be exactly 140 mmol.
The reaction follows a one-to-one ratio between NOCl, NO, and Cl.
The maximum amount of NOCl that can be formed is 140 mmol.
The problem tests understanding of chemical equilibrium and reaction stoichiometry.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: