Periodic Table Explained: Name Origin
TLDRThis script explores the fascinating world of the periodic table, detailing how scientists identified 92 elements, each with unique properties. Elements are ordered by atomic weight, with each assigned an atomic number. The video highlights the periodic nature of the table, showing how elements transition from inert gases to soft, shiny metals, then to harder metals, semimetals, and nonmetals. It explains the pattern of elements and their properties, such as reactivity and conductivity, and how these patterns led to the creation of the periodic table's seven horizontal rows, each ending with an inert gas. The script provides an engaging and educational overview of the periodic table's structure and significance.
Takeaways
- 🌌 There are 92 different kinds of atoms, each constituting a unique element.
- 🔬 Scientists have categorized elements by their atomic weights, starting with hydrogen, the lightest, to uranium, the heaviest.
- 🔢 Each element has an atomic number that reflects its position in the list, with hydrogen at one and uranium at ninety-two.
- 🔥 Hydrogen is chemically reactive and can explode when ignited, unlike helium, which is chemically inert and extinguishes flames.
- 🌟 Following the inert helium, there's a transition to metals like lithium and beryllium, which are soft and shiny.
- 🛠 Metals are generally malleable, shiny, and conductive solids, with a few exceptions like silicon, which is a semi-metal.
- 💨 Nonmetals, often found towards the right of the periodic table, are gases that do not conduct electricity, such as phosphorus, sulfur, chlorine, and argon.
- 💤 Inert gases like neon, argon, krypton, and radon are colorless and do not react chemically.
- 🔄 The periodic table shows a pattern where each cycle ends with an inert gas and begins with a soft, shiny metal.
- 📊 The periodic table is organized into seven horizontal rows, each ending with an inert gas and starting with a metal.
- 📚 The arrangement of elements in rows and columns is periodic, hence the name 'periodic table,' reflecting the repetition of properties.
Q & A
How many different kinds of atoms, or elements, are there in the universe according to the script?
-There are 92 different kinds of atoms, or elements, in the universe.
What is the term used to describe a substance made of only one kind of atom?
-A substance made of only one kind of atom is called an element.
How did scientists categorize the elements based on the weight of their atoms?
-Scientists made a list starting with the lightest element, hydrogen, to the heaviest, uranium, based on the weights of their atoms.
What is the term for the number that represents an element's position in the list of elements ordered by atomic weight?
-The number that represents an element's position in the list is called the atomic number.
Why does hydrogen explode when a lighted match is placed to it, while helium just extinguishes the flame?
-Hydrogen is chemically reactive, whereas helium is chemically unreactive or inert.
What is the general property of metals as described in the script?
-Metals are usually shiny, malleable solids that can conduct electricity.
How does the script describe the transition from metals to nonmetals in the periodic table?
-The script describes a transition from metals to nonmetals, with elements like silicon being semimetals and less conductive, followed by nonmetals that do not conduct electricity at all.
What is the significance of inert colorless gases in the periodic table?
-Inert colorless gases, such as helium, neon, and argon, are chemically unreactive and mark the end of each row in the periodic table.
Why are the breaks in the periodic table getting further apart as we move down the list?
-The breaks are getting further apart because there are more metals and fewer nonmetals between the breaks, indicating a pattern in the arrangement of elements.
What is the term used to describe the repetitive pattern observed in the arrangement of elements in the periodic table?
-The repetitive pattern observed in the arrangement of elements is referred to as 'periodic', which is also part of the name 'periodic table'.
How did scientists create the seven horizontal rows in the periodic table?
-Scientists divided the long line of elements into seven shorter lines at the breaks where an inert colorless gas meets a soft shiny metal, thus creating the seven horizontal rows.
Outlines
🌌 Elements and the Periodic Table
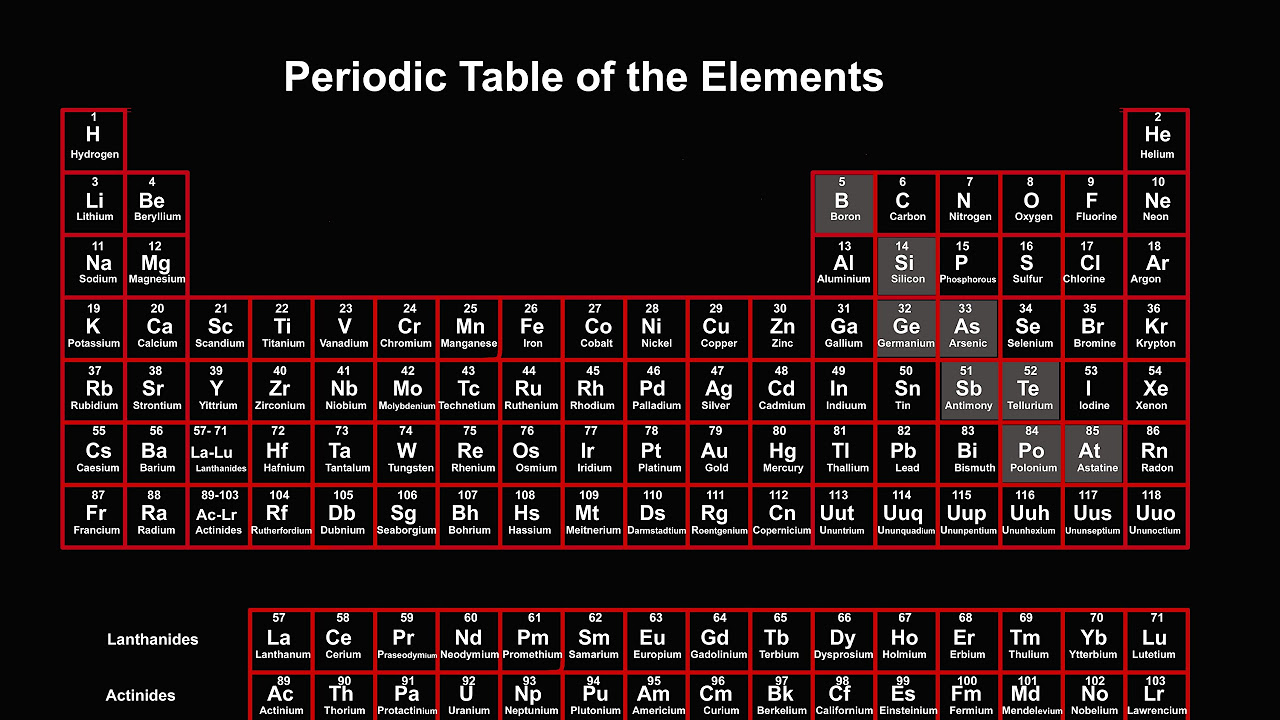
This paragraph introduces the concept of elements, which are pure substances made up of only one kind of atom. It explains that there are 92 known elements in the universe, each with a unique atomic number that reflects its atomic weight. The paragraph describes the properties of the first few elements in the periodic table, highlighting the differences between reactive elements like hydrogen and inert gases like helium. It also discusses the transition from metals to nonmetals and back to metals, emphasizing the periodic nature of these properties, which is the basis for the periodic table's structure.
🔍 The Structure of the Periodic Table
The second paragraph delves into the structure of the periodic table, explaining how scientists have organized the elements into rows and columns based on their properties. It describes the significance of inert gases as the end of each row and the start of a new one, and how these elements are followed by soft, shiny metals, indicating the beginning of a new cycle. The paragraph also discusses the increasing distance between these breaks and the corresponding increase in the number of metals compared to nonmetals. It concludes by explaining how the periodic table is arranged with metals on the left, semimetals in the middle, and nonmetals on the right, with each row ending in an inert gas, thus reflecting the periodic nature of the elements' properties.
Mindmap
Keywords
💡Atoms
💡Elements
💡Atomic Number
💡Chemical Reactivity
💡Inert Gases
💡Metals
💡Semimetals
💡Nonmetals
💡Periodic Table
💡Periodicity
Highlights
Discovery of 92 different kinds of atoms that exist in the universe, each kind forming a unique element.
Scientists created a list of elements based on atomic weights, ranging from lightest hydrogen to heaviest uranium.
Introduction of atomic number, a unique number assigned to each element based on its atomic weight.
Hydrogen has an atomic number of one, and uranium has an atomic number of 92.
Chemical reactivity differences between hydrogen and helium, despite their similar appearance.
Helium is chemically unreactive or inert, unlike reactive hydrogen.
Lithium and beryllium are metals with different hardness levels.
Boron is a brown powder, contrasting with the shiny metals to its left.
Neon is a colorless gas that is chemically unreactive, similar to helium.
Sodium, magnesium, and aluminum are harder metals known for their conductivity.
Silicon is a semi-metal with less conductivity compared to metals.
Phosphorus, sulfur, chlorine, and argon are nonmetals with varying states of matter.
Argon is another inert gas, highlighting a pattern in the periodic table.
The periodic table's structure is based on the repetitive nature of element properties.
Inert gases and soft shiny metals mark the beginning of new rows in the periodic table.
The periodic table is divided into seven horizontal rows, each ending with an inert gas.
Each row in the periodic table follows a pattern of metals, semimetals, and nonmetals.
The last colorless inert gas on the list is radon, with elements beyond it being rare and unobservable.
Uranium concludes the list of observable elements in the periodic table.
Transcripts
Browse More Related Video

Periodic Table of Elements Song

Periodic Table of Elements Explained - Metals, Nonmetals, Valence Electrons, Charges
Periodic Table Explained: Introduction

Periodic Table of Elements - Element Classes

The Periodic Table of the Elements in Chemistry - [1-2-12]

The periodic table | Atoms, elements, and the periodic table | High school chemistry | Khan Academy
5.0 / 5 (0 votes)
Thanks for rating: