Chemistry Reference table C
TLDRThis five-minute educational video introduces Reference Table C, a tool for chemistry students to understand and perform dimensional analysis with metric units. The instructor emphasizes the importance of quantifying matter in chemistry and guides viewers through the process of converting units, such as from kilograms to grams, using conversion factors. The video also touches on scientific notation and the significance of prefixes in unit conversion, aiming to simplify complex numbers for better comprehension.
Takeaways
- 📚 The video is about Reference Table C, which is used for chemistry measurements and understanding matter.
- 📈 The script introduces the concept of 'quant' which relates to quantifying matter through numbers and math.
- 🔍 Dimensional analysis is a key technique that will be emphasized in the video, important for converting between units.
- 🌐 The focus is on metric units, not conversions between metric and imperial systems or temperature scales.
- 🔢 Reference Table C includes factors and prefixes that are crucial for understanding large and small quantities in chemistry.
- 📏 The prefix 'kilo' means a thousand, and it's used to move the decimal point three places to the right in scientific notation.
- 📉 Conversely, the prefix 'deci' means one-tenth, requiring a decimal point move to the left in scientific notation.
- 📘 An example problem is provided to demonstrate converting .0089 kilograms to grams using dimensional analysis.
- 📝 The importance of reporting scientific information in a comprehensible format is highlighted to give a better mental image of the size of numbers.
- 🖊️ The script mentions the use of technology for recording and note-taking, showing an adaptation to new tools during challenging times.
- 🗓️ There's an anticipation of meeting students in September, indicating the video is likely recorded in preparation for an upcoming academic year.
Q & A
What is the main purpose of the video?
-The main purpose of the video is to explain Reference Table C, which will be used for teaching chemistry concepts involving measurement and dimensional analysis at the beginning of the school year.
Why is dimensional analysis important in chemistry?
-Dimensional analysis is important in chemistry because it helps in converting between different units of measurement, which is essential for quantifying matter and performing calculations in chemical equations.
What does the prefix 'kilo' signify in the metric system?
-The prefix 'kilo' in the metric system signifies a thousand (10^3), indicating a large quantity or measurement.
How does the video instructor relate the prefix 'kilo' to everyday life?
-The instructor relates the prefix 'kilo' to everyday life by using the example of a crime TV show where someone gets caught with a kilo of a drug, emphasizing that a kilo represents a significant amount.
What is the relationship between kilograms and meters as explained in the video?
-In the video, it is explained that one kilometer is equal to a thousand meters, demonstrating the conversion between units of length using the metric system.
What is the purpose of the example problem with .0089 kilograms?
-The purpose of the example problem is to illustrate how to convert a small quantity in kilograms to grams using dimensional analysis, making it easier to understand the size of the number.
How does the instructor suggest converting scientific notation to a more familiar number?
-The instructor suggests converting scientific notation, such as 10^3, to a more familiar number by recognizing it as a thousand, which is easier for most people to comprehend.
What is the significance of the instructor mentioning 'covent spring'?
-The mention of 'covent spring' refers to the period during which the instructor has been adapting to new technology for teaching, possibly due to the COVID-19 pandemic, indicating a shift to online or remote learning.
What is the main focus of the first unit in chemistry according to the video?
-The main focus of the first unit in chemistry is on matter and how to measure it, emphasizing the importance of quantifying matter in the study of chemistry.
Why does the instructor recommend re-watching the video if dimensional analysis is not understood immediately?
-The instructor recommends re-watching the video because dimensional analysis is a fundamental concept that may not be easily grasped by everyone, and repeated viewings can help reinforce understanding.
What metric units are mentioned in the video for measuring length and volume?
-The metric units mentioned in the video for measuring length are meters, and for measuring volume, liters are used.
Outlines
📚 Introduction to Reference Table C for Chemistry
The video begins with a warm welcome and an introduction to Reference Table C, which is designed to be used at the start of the academic year to assist with chemistry-related measurements. The instructor emphasizes the importance of quantifying matter in chemistry, which involves numbers and mathematical operations. The video promises to cover dimensional analysis, a technique that will be frequently used in school. The instructor also shares their personal experience of adjusting to new technology for recording the video, hinting at the ongoing challenges and learning curve associated with it.
🔍 Understanding the Metric System and Dimensional Analysis
This paragraph delves into the specifics of the metric system, which is the primary focus of Reference Table C. The instructor explains that the metric system is used for measuring length and volume, with units such as meters and grams. The concept of prefixes within the metric system is introduced, with 'kilo' meaning a thousand, and the instructor uses the example of a kilogram to illustrate how to convert between units using scientific notation. The paragraph also introduces the concept of dimensional analysis, which involves writing down the number and units, and then using a conversion factor to change from one unit to another. The instructor provides a step-by-step example of converting kilograms to grams, emphasizing the importance of moving the decimal point correctly based on the exponent in scientific notation.
Mindmap
Keywords
💡Reference Table C
💡Chemistry
💡Quantification
💡Dimensional Analysis
💡Metric System
💡Prefixes
💡Scientific Notation
💡Conversion Factor
💡Meters and Grams
💡Example Problem
Highlights
Introduction to a quick five-minute video on reference table C.
Reference table C is enlarged for better visibility of handwriting.
Video is recorded in July with an anticipation to meet the audience in September.
Reference table C will be used for chemistry measurements in the beginning of the year.
Chemistry is the study of matter and involves quantifying it.
Dimensional analysis will be a key focus in the chemistry curriculum.
The importance of understanding dimensional analysis is emphasized for those who find it difficult initially.
The presenter is adapting to new technology for recording videos.
Conversions will be limited to the metric system, excluding Fahrenheit to Celsius conversions.
Reference table D contains selected metric units for length and mass.
Explanation of metric prefixes and their meanings, such as 'kilo' for a thousand.
Conversion from scientific notation to a more understandable format for large numbers.
Demonstration of converting .0089 kilograms to grams using dimensional analysis.
The process of writing down the number and units before applying the conversion factor.
Using the conversion factor of 10 to the power 3 grams per kilogram.
Alternative to scientific notation by converting 10 to the power 3 to a thousand.
Final remarks on the mini-lesson focusing on reference table C for chemistry measurements.
Transcripts
Browse More Related Video
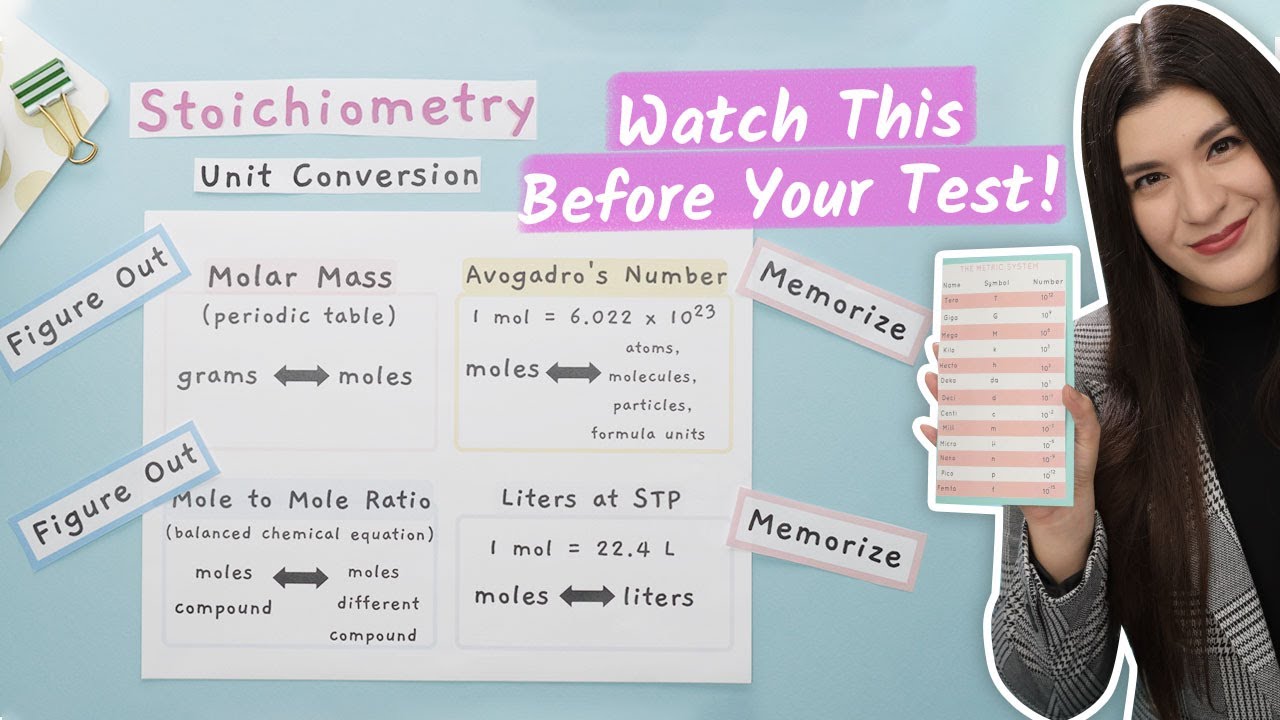
How to Convert Units in Chemistry

Metric Units of Mass | Convert mg, g, and kg
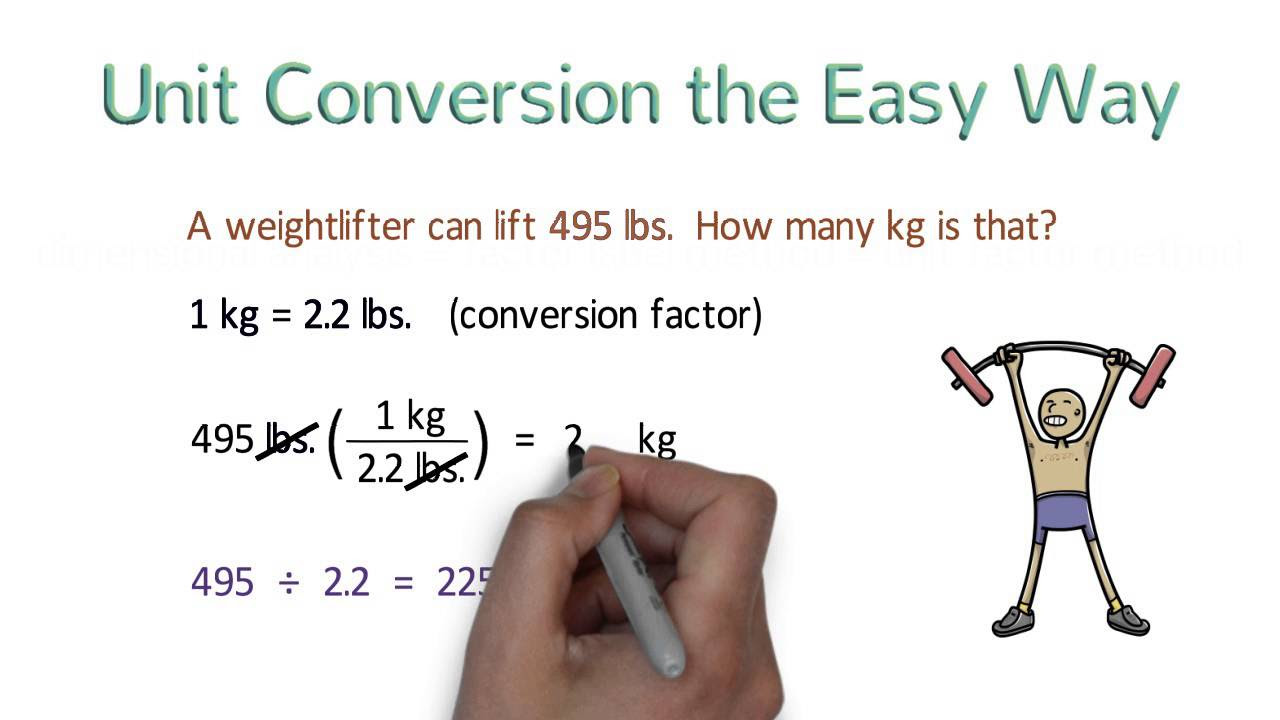
Unit Conversion the Easy Way (Dimensional Analysis)

02 - Learn Unit Conversions, Metric System & Scientific Notation in Chemistry & Physics
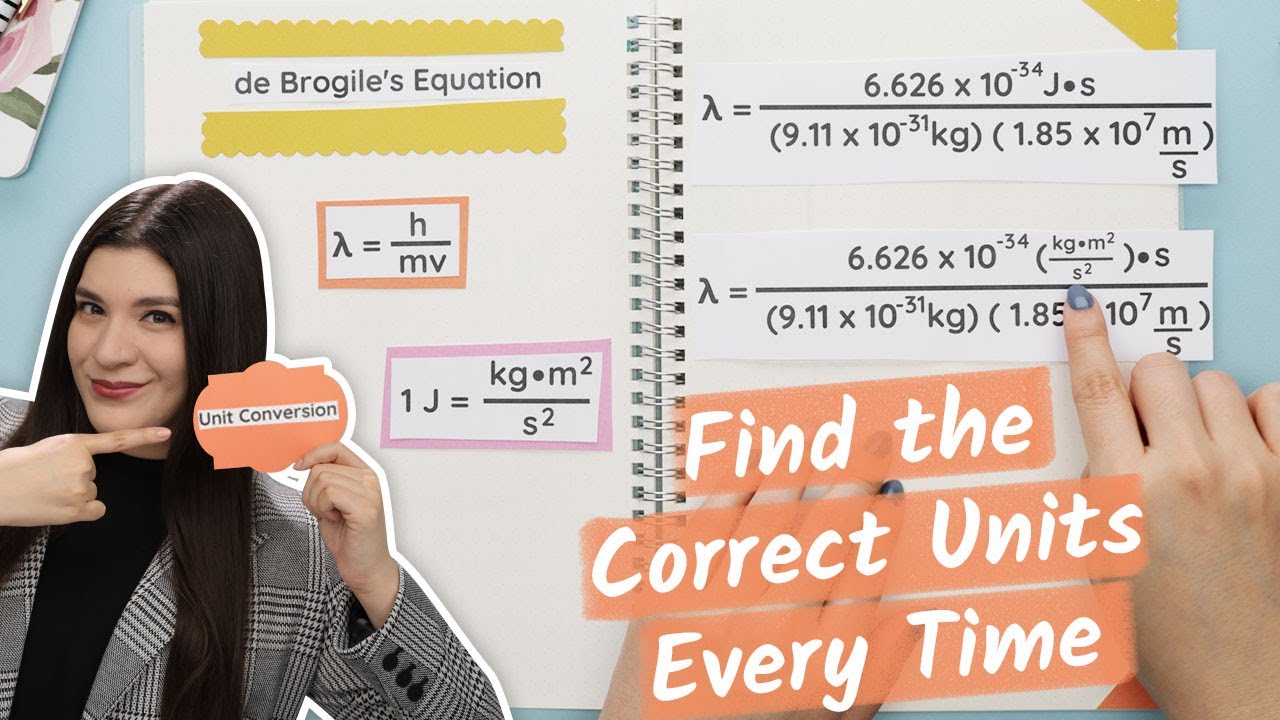
How to Determine Your Units in Chemistry

2.4 Units and Conversions | High School Chemistry
5.0 / 5 (0 votes)
Thanks for rating: