Covalent networks, metallic crystals, and ionic crystals | Chemistry | Khan Academy
TLDRThis video explores the strength of different crystal structures, starting with the covalent network exemplified by diamond, which is incredibly strong due to its extensive covalent bonding. It then discusses ionic crystals like sodium chloride, highlighting their brittleness and high boiling points, and metallic crystals, which conduct electricity well and vary in hardness. The video concludes by emphasizing the importance of intermolecular bonds in determining a substance's properties, such as boiling points and strength.
Takeaways
- 🔬 The strongest structure among crystals is the covalent network, exemplified by diamond, where carbon atoms form a continuous network of covalent bonds.
- 💎 Diamonds are extremely strong and have a high boiling point due to the extensive covalent bonding throughout the entire structure.
- 🧊 Ice is a type of crystal that forms a regular structure due to hydrogen bonding when the temperature is low enough.
- 🔍 Crystals are solids where the constituent molecules are arranged in a regular, consistent pattern, contrasting with amorphous solids which lack a regular structure.
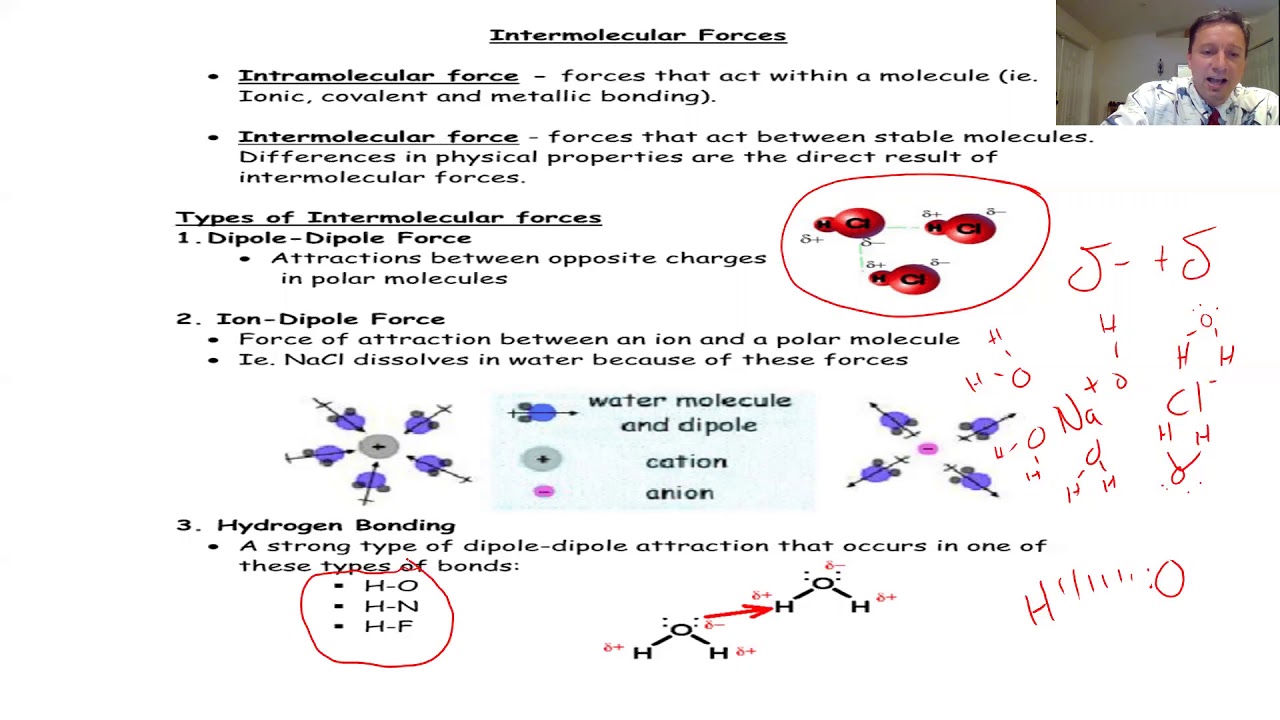
- ⚜️ Ionic crystals, like sodium chloride, have a strong bond due to the electrostatic attraction between positively and negatively charged ions, making them brittle.
- 🌊 Metallic crystals consist of metal atoms that share their valence electrons freely, creating a 'sea of electrons' that contributes to their conductivity and malleability.
- 🌟 The strength and boiling point of a substance are related to the strength of the intermolecular bonds it possesses.
- 💧 Ionic crystals can dissolve in water, forming ionic dipoles that allow the substance to become conductive due to the movement of charged particles.
- 🌀 The dissolution of ionic crystals in water involves the ions being attracted to oppositely charged ends of water molecules, facilitating the dissolution process.
- 📉 The strength and boiling point of substances can vary widely, from very hard and high boiling points in covalent and ionic crystals to softer and lower boiling points in some metals.
- 🔑 Understanding the type of bonds and structures in a substance can help predict its physical properties, such as strength, boiling point, and state of matter.
Q & A
What is the strongest type of crystal structure?
-The strongest type of crystal structure is the covalent network, as exemplified by diamond, where carbon atoms form four covalent bonds with each other, creating an extremely strong and stable structure.
How does the structure of a covalent network contribute to the properties of a diamond?
-In a covalent network like that of a diamond, each carbon atom forms four covalent bonds with other carbon atoms, creating a continuous network. This results in the diamond being extremely strong, hard, and having a very high boiling point.
What is the difference between a crystal and an amorphous solid?
-A crystal is a solid where the molecules are arranged in a regular, consistent pattern, while an amorphous solid lacks this regular arrangement, having a more random and disordered structure.
Why are ionic crystals considered strong in terms of their physical properties?
-Ionic crystals are strong because the ions are held together by ionic bonds, which are formed when one atom donates an electron to another. This results in a lattice structure that is quite brittle but can have a high boiling point.
How does the structure of a metallic crystal differ from that of an ionic crystal?
-In a metallic crystal, atoms share their valence electrons, creating a 'sea' of free electrons that allows for good electrical conductivity. This differs from ionic crystals, where electrons are transferred to form ions that are held together by electrostatic attraction.
What happens when an ionic crystal like sodium chloride is dissolved in water?
-When sodium chloride is dissolved in water, the ionic bonds are disrupted, and the ions become surrounded by water molecules, forming ionic dipole bonds. This allows the salt to dissolve and the solution to conduct electricity.
Why are metals generally good conductors of electricity?
-Metals are good conductors of electricity because they have a 'sea' of free electrons that can move easily through the crystal lattice, allowing for the flow of electric current.
How does the strength of atomic bonds relate to the hardness or brittleness of a material?
-The strength of atomic bonds directly influences the hardness or brittleness of a material. Stronger bonds typically result in harder and more brittle materials, while weaker bonds can lead to softer and more flexible materials.
What is the significance of the boiling point in relation to the strength of bonds in a substance?
-A higher boiling point generally indicates stronger bonds within a substance. Substances with strong bonds, like covalent networks or ionic crystals, require more energy to break these bonds and reach their boiling point.
How can the properties of a substance be inferred from its intermolecular forces?
-The properties of a substance, such as its boiling point and strength, can be inferred from the type of intermolecular forces present. Stronger forces, like covalent bonds, lead to higher boiling points and greater strength, while weaker forces, like London dispersion forces, result in lower boiling points and less strength.
Outlines
💎 Covalent Network Crystals and Diamond's Strength
This paragraph introduces the concept of covalent network crystals, which are the strongest type of crystal structures. It explains that in these structures, atoms are bonded by sharing electrons, creating a consistent and regular pattern. The prime example given is diamond, formed by carbon atoms each sharing electrons to form four covalent bonds with other carbon atoms. The entire diamond is essentially one giant molecule, making it extremely strong and difficult to break. This covalent bonding is the strongest type of molecular bond, contributing to diamond's high boiling point and hardness.
🔬 Ionic and Metallic Crystals: Structure and Properties
The second paragraph delves into ionic and metallic crystals, contrasting their properties and bonding mechanisms. Ionic crystals, exemplified by sodium chloride, involve the transfer of electrons from metal to non-metal atoms, forming positively and negatively charged ions that are attracted to each other. This creates a strong bond, resulting in a brittle and high-boiling-point structure. Metallic crystals, on the other hand, involve a 'sea' of delocalized electrons that can move freely, making metals good conductors of electricity. Metals can be hard or soft, and their strength often comes from their ability to bend or flex, which allows them to deflect force. The paragraph also discusses how ionic crystals dissolve in water to form ionic dipoles, which can conduct electricity due to the movement of charged particles.
Mindmap
Keywords
💡Covalent Network
💡Crystal
💡London Dispersion Force
💡Ionic Crystal
💡Metallic Crystal
💡Boiling Point
💡Dipole-Dipole Forces
💡Amorphous Solid
💡Valence Electrons
💡Ionic Dipole Bonds
💡Intermolecular Bonds
Highlights
The weakest intermolecular force is the London dispersion force.
Covalent network is the strongest crystal structure.
A crystal is defined as a solid with a regular, consistent pattern of molecules.
Amorphous solids lack the regular structure found in crystals.
Ice forms a crystal structure due to hydrogen bonds at low temperatures.
Diamond is an example of a covalent network crystal, where carbon atoms form four bonds with each other.
The entire diamond can be viewed as one molecule due to its covalent bonds.
Covalent bonds are the strongest type of molecular bonds.
Diamond's high boiling point and strength are due to its covalent network structure.
Ionic crystals, like sodium chloride, involve the transfer of electrons to form positive and negative ions.
Ionic bonds are strong and can result in high boiling points.
Ionic crystals can be brittle, as demonstrated by the ease of breaking a block of table salt.
Metallic crystals involve the sharing of electrons among metal atoms, forming a sea of electrons.
Metals can be both hard and soft, depending on the type, with gold being an example of a soft metal.
Metallic crystals can be ductile, allowing them to bend or flex and absorb force.
Ionic crystals can dissolve in water, forming ionic dipole bonds with water molecules.
Dissolving ionic crystals in water makes the solution conductive due to the movement of charged particles.
The strength and boiling point of a substance can be inferred from the type of intermolecular bonds it has.
A single molecular structure, like in diamonds, results in extremely strong bonds.
Gases like neon have very weak bonds due to only London dispersion forces.
Transcripts
Browse More Related Video

AP Chemistry Unit 2 Review: Compound Structure and Properties (includes dot structure stuff :D)
AP Chemistry Unit 2 Review

What Are Covalent Bonds | Properties of Matter | Chemistry | FuseSchool

How Do Atoms Bond - Part 2 | Properties of Matter | Chemistry | FuseSchool

AP Chem - Unit 3 Review - Intermolecular Forces & Properties

Ionic bonds and Coulombs law
5.0 / 5 (0 votes)
Thanks for rating: