15.1 Chemical Equilibrium and Equilibrium Constants | General Chemistry
TLDRIn this informative lesson, Chad from Chad's Prep delves into the concepts of chemical equilibrium and equilibrium constants. He explains that equilibrium is a dynamic state where the concentrations of reactants and products remain constant, not because the reaction stops, but because the forward and reverse reactions occur at the same rate. Chad further clarifies the difference between Kc and Kp, emphasizing that the equilibrium constant is dependent solely on temperature and is always unitless. Through examples, he illustrates how the value of the equilibrium constant changes with reaction manipulation, such as reversing or halving the reaction. The lesson is a valuable resource for students seeking to understand and apply these fundamental chemistry concepts.
Takeaways
- 🌟 Equilibrium in chemistry refers to a dynamic state where the concentrations of reactants and products are no longer changing because the forward and reverse reactions occur at the same rate.
- 📈 The equilibrium constant (K) is symbolized by a capital K and represents the ratio of the concentrations of products to the concentrations of reactants raised to the power of their coefficients.
- 🔥 The value of K is unitless and only changes with temperature, not with the addition of a catalyst or changes in activation energy.
- 🧪 For reactions involving gases, two types of equilibrium constants can be defined: Kc (based on molar concentrations) and Kp (based on partial pressures), which are not necessarily equal.
- 📊 The equilibrium constant provides insight into whether a reaction favors the formation of products or reactants, with a large K value (>>1) indicating a product-favored reaction and a small K value (<<1) indicating a reactant-favored reaction.
- 🔢 The concentrations used to calculate K must be those at equilibrium; the reaction quotient is used for systems that have not yet reached equilibrium.
- 🛠️ The relationship between Kc and Kp for a given reaction can be expressed as Kp = Kc * (RT/Δn)^Δn, where Δn is the change in the number of moles of gas and R is the ideal gas constant.
- 🔄 For related reactions, the equilibrium constant changes depending on the reaction direction and coefficients. Reversing a reaction inverts the K value, and changing coefficients affects the K value according to the roots of the new coefficients.
- 📚 The equilibrium constant is a powerful tool for predicting the extent of a reaction and can inform industrial processes aiming to maximize the production of certain chemicals.
- 🎓 Understanding the relationships between different reactions and their equilibrium constants is crucial for problem-solving in chemistry, especially in predicting the outcomes of related reactions with altered coefficients or direction.
- 🚀 The concept of equilibrium and equilibrium constants is fundamental to the study of chemical reactions, particularly in the context of general chemistry and related scientific disciplines.
Q & A
What is the definition of dynamic equilibrium in chemistry?
-Dynamic equilibrium in chemistry refers to a state where the concentrations of reactants and products are no longer changing because the forward and reverse reactions are occurring at the same rate, even though the reactions have not stopped.
What is a misconception about concentrations at equilibrium?
-A common misconception is that the concentrations of reactants and products must be equal at equilibrium. However, this is not necessarily true. The concentrations at equilibrium can vary, and it is the rates of the forward and reverse reactions that are equal, not the concentrations.
What is the equilibrium constant (K) and how is it represented?
-The equilibrium constant (K) is a measure of the extent to which a reaction proceeds at equilibrium. It is represented by the letter K (capital K to distinguish it from the rate constant, k) and is calculated as the ratio of the concentrations of products to the concentrations of reactants, each raised to the power of their respective coefficients in the balanced chemical equation.
How does temperature affect the equilibrium constant?
-The equilibrium constant is only affected by changes in temperature. Adding a catalyst or changing the activation energy does not change the equilibrium constant. An increase in temperature will either increase or decrease the value of K, depending on whether the reaction is endothermic or exothermic.
What are Kc and Kp values and when are they used?
-Kc and Kp are two types of equilibrium constants. Kc is used for reactions involving only aqueous species and is based on molar concentrations. Kp is used for reactions involving gases and is based on partial pressures. These values are not necessarily equal, and their use depends on the phase of the substances involved in the reaction.
What does the value of the equilibrium constant indicate about a reaction?
-The value of the equilibrium constant can indicate whether a reaction favors the formation of products or reactants at equilibrium. If K is much greater than 1, the reaction favors the formation of products. If K is much less than 1, the reaction favors the formation of reactants. If K is close to 1, there is a significant amount of both reactants and products at equilibrium.
How are Kc and Kp related and when would they be equal?
-Kc and Kp are related through the equation Kp = Kc * (RT/Δn)^Δn, where R is the gas constant, T is the temperature in Kelvin, and Δn is the change in the number of moles of gas from reactants to products. Kp and Kc would be equal if Δn is zero, meaning there is no change in the number of moles of gas between reactants and products.
What is the significance of the units of the equilibrium constant?
-The equilibrium constant is always unitless. This is because it is a ratio of concentrations (or pressures) that are measured at equilibrium and are therefore dimensionless when expressed per unit concentration or per unit pressure.
How does changing the stoichiometry of a reaction affect its equilibrium constant?
-Changing the stoichiometry of a reaction affects the equilibrium constant by altering the powers to which the concentrations (or pressures) are raised. If the reaction is doubled (coefficients multiplied by 2), the equilibrium constant is squared. If the reaction is halved (coefficients multiplied by 1/2), the equilibrium constant takes the square root of the original value. If the reaction is reversed, the equilibrium constant is the inverse of the original value.
What is the relationship between the equilibrium constant of a reaction and its reverse?
-The equilibrium constant of a reaction and its reverse are reciprocals of each other. If the equilibrium constant for the forward reaction is K, then the equilibrium constant for the reverse reaction is 1/K.
How can you determine if a reaction will favor the formation of products or reactants without calculating the equilibrium constant?
-You can determine if a reaction will favor the formation of products or reactants by looking at the balanced chemical equation. If there are more moles of gas on the product side than the reactant side, the reaction will likely favor the formation of products. Conversely, if there are more moles of gas on the reactant side, the reaction will likely favor the formation of reactants.
Outlines
📚 Introduction to Equilibrium and Equilibrium Constants
The video begins with an introduction to the concepts of equilibrium and equilibrium constants, emphasizing the dynamic nature of equilibrium in chemical reactions. The instructor, Chad, outlines the scope of the lesson, which includes understanding the definition of equilibrium, the distinction between static and dynamic equilibrium, and the misconception that equilibrium implies equal concentrations of reactants and products. The lesson also introduces the equilibrium constant (K), its relationship with reaction rates, and how it is affected by temperature and catalysts. The importance of measuring concentrations at equilibrium for calculating K is highlighted, along with the exclusion of solids and liquids from equilibrium expressions due to their activity of one.
🧪 Calculation of Equilibrium Constants (Kc and Kp)
This paragraph delves into the calculation of equilibrium constants, differentiating between Kc (based on molar concentrations) and Kp (based on partial pressures). The video explains the ideal gas law and how it relates to the difference between Kc and Kp values. It clarifies that while Kc and Kp are not necessarily equal, they can be under certain conditions. The lesson also addresses the improper use of the term 'Keq' in general chemistry and its proper usage in advanced courses. The importance of temperature in determining the value of equilibrium constants is reiterated, with a focus on how equilibrium constants can indicate the extent of product formation in a reaction.
📈 Interpreting Equilibrium Constants: Favoring Reactants or Products
The video segment discusses the significance of the magnitude of equilibrium constants in predicting the outcome of a reaction. It outlines three scenarios based on the value of the equilibrium constant: greatly大于 one, significantly小于 one, and close to one. A large equilibrium constant (e.g., 10^9) indicates that a reaction strongly favors the formation of products, while a small equilibrium constant (e.g., 10^-10) suggests that the reaction favors the reactants. An equilibrium constant close to one implies a balance between reactants and products. The video also touches on the implications of these constants for industrial chemical production and how they can guide the design of manufacturing processes.
🔢 Relationship Between Kc and Kp: Calculations and Principles
This part of the lesson focuses on the relationship between Kc and Kp, explaining how changes in the number of moles of gas during a reaction affect these values. The video presents the formula Kp = Kc * (RT/Δn)^Δn, where Δn is the change in moles of gas. It clarifies that Kc and Kp will only be equal if Δn is zero, which occurs when the number of moles of gas is the same on both sides of the reaction. The lesson also addresses the unitless nature of equilibrium constants and contrasts this with rate constants, which have units dependent on the reaction order. The video provides a practical example of calculating Kp from a given Kc value, incorporating temperature and the ideal gas constant.
🔄 Understanding the Effects of Reaction Changes on Equilibrium Constants
The final paragraph explores how altering a reaction's stoichiometry affects its equilibrium constant. It explains that reversing a reaction inverts the equilibrium constant, and changing the coefficients (e.g., cutting them in half) results in taking the square root of the constant. The video provides examples of how these changes impact the calculated Kc values, emphasizing the importance of understanding these relationships for problem-solving in chemistry. It also offers strategies for quickly determining the equilibrium constant of related reactions without extensive calculations, which can be beneficial during exams.
Mindmap
Keywords
💡Equilibrium
💡Equilibrium Constant (K)
💡Dynamic Equilibrium
💡Concentrations
💡Reactants and Products
💡Molar Concentrations
💡Partial Pressures
💡Stoichiometric Coefficients
💡Temperature
💡Reaction Quotient
Highlights
Equilibrium and equilibrium constants are the main topics of the lesson.
Dynamic equilibrium is when the concentrations of reactants and products are no longer changing because the forward and reverse reactions occur at the same rate.
At equilibrium, the concentrations of reactants and products do not have to be equal.
The equilibrium constant (K) is only affected by temperature changes and is independent of catalysts or activation energy.
The equilibrium constant is the ratio of the concentrations of products to the concentrations of reactants, raised to the power of their coefficients.
Solids and liquids are typically not included in the equilibrium constant expression because they have an activity of one.
There are two types of equilibrium constants: Kc (for molar concentrations) and Kp (for partial pressures).
The equilibrium constant can indicate whether a reaction favors products, reactants, or both equally.
The value of an equilibrium constant can be used to predict the extent of a reaction and its efficiency in industrial processes.
The relationship between Kc and Kp for a reaction involving gases can be described by the equation Kp = Kc * (RT/Δn)^Δn, where Δn is the change in the number of moles of gas.
Equilibrium constants are always unitless, unlike rate constants which have units based on the reaction order.
The value of the equilibrium constant changes if the reaction is reversed or the coefficients are altered.
For a reversed reaction, the equilibrium constant is the inverse of the original reaction's constant.
If the coefficients of a reaction are halved, the equilibrium constant is the square root of the original constant.
For a reaction that is both reversed and halved, the equilibrium constant is the inverse of the original, taken to the square root.
Understanding the relationships between equilibrium constants is crucial for solving problems on chemical equilibria.
The lesson provides practical insights into how equilibrium constants can be applied in industrial settings to optimize chemical production.
Transcripts
Browse More Related Video
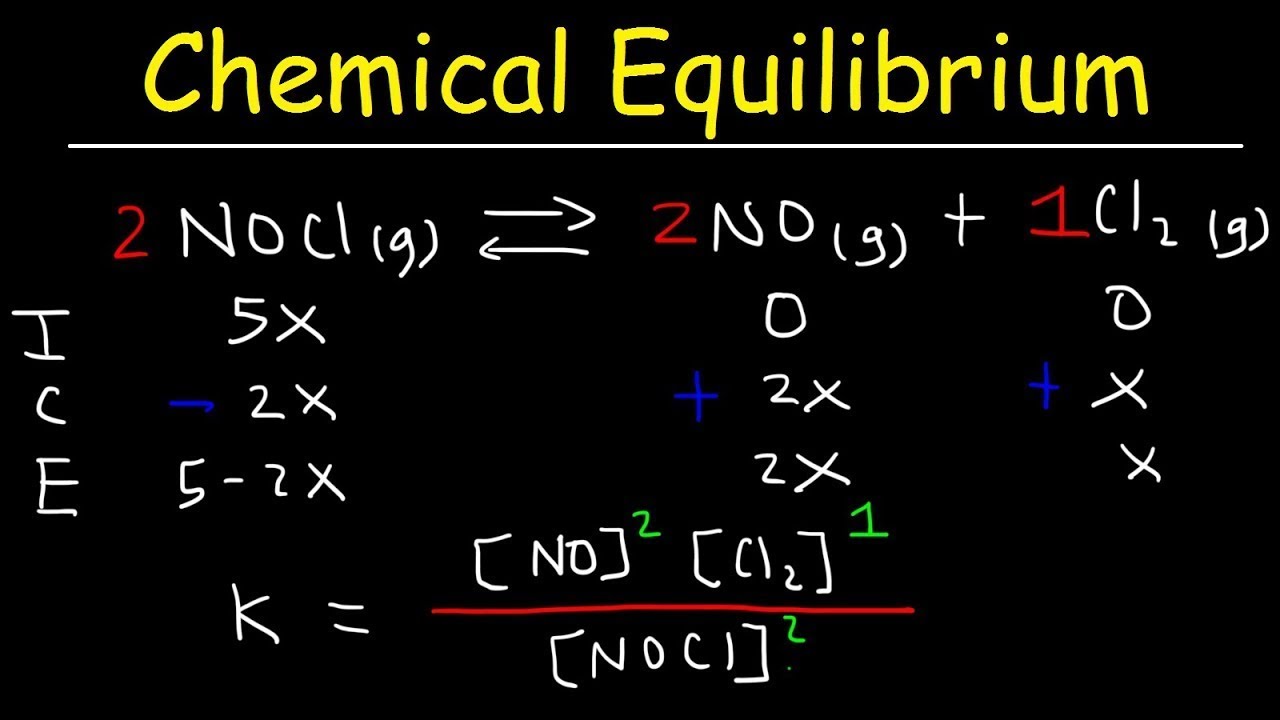
Chemical Equilibrium Constant K - Ice Tables - Kp and Kc

Chemical Equilibria and Reaction Quotients

AP Chemistry Unit 7 Review: Equilibrium!

Chemistry_Calculating the Equilibrium constant
What Is Equilibrium? AP Chemistry Unit 7, Topic 1 Daily Video

18.3 Gibbs Free Energy and the Relationship between Delta G, Delta H, & Delta S | General Chemistry
5.0 / 5 (0 votes)
Thanks for rating: