Periodic Trends: Ionization Energy Explained With Exceptions | Study Chemistry With Us
TLDRThis educational video script delves into the concept of ionization energy, a crucial topic in chemistry. It explains ionization energy as the energy required to remove an electron from a gaseous atom, emphasizing the periodic trend where ionization energy increases across a period and decreases down a group. The script also addresses exceptions to the trend, particularly in groups 2A and 6A, and the higher energy needed to remove subsequent electrons. It encourages students to understand these trends and exceptions to succeed in exams, offering practical advice on memorization and problem-solving.
Takeaways
- 📚 Ionization energy is the energy required to remove an electron from an atom in its gaseous state.
- 🔄 The trend for ionization energy is the opposite of atomic or ionic radii; it increases across a period (left to right) and decreases down a group.
- ⚛️ The first ionization energy is the focus of the trend and is the lowest energy needed to remove one electron from a neutral atom.
- 🔗 Ionization energy is directly related to the effective nuclear charge (Z effective), which influences how strongly an electron is attracted to the nucleus.
- 🌟 There are exceptions to the ionization energy trend, particularly with groups 2A and 5A having higher ionization energies than their adjacent groups.
- 💡 Full and half-full orbitals are more stable and thus require more energy to remove an electron, which is why Be has a higher first ionization energy than B, and N has a higher first ionization energy than O.
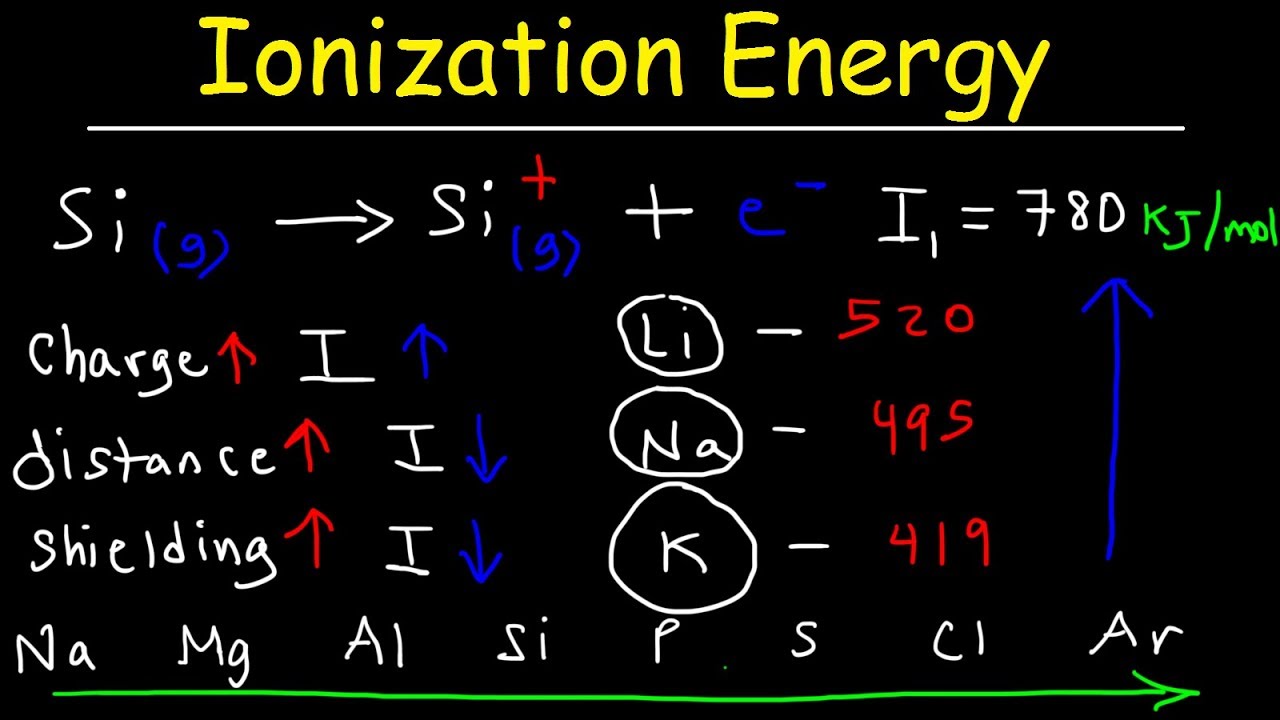
- 📉 Moving down a group, the ionization energy decreases because valence electrons are further from the nucleus and easier to remove.
- 📈 Moving across a period, the ionization energy increases due to the increase in effective nuclear charge, making it harder to remove an electron.
- 🔢 Higher ionization energies (second, third, etc.) require more energy to remove subsequent electrons from an atom.
- 📝 It's important to understand both the trend and the exceptions to predict ionization energies correctly.
- 📚 Memorizing exceptions and understanding electron configurations can be a strategy for tackling ionization energy problems.
Q & A
What is ionization energy?
-Ionization energy is the energy required to remove an electron from an atom in its gaseous state, resulting in the formation of a cation.
Why is it important to understand the trend of ionization energy across the periodic table?
-Understanding the trend of ionization energy helps predict the ease with which an electron can be removed from an atom, which is crucial for various chemical reactions and processes.
What is the general trend of ionization energy as you move from left to right across the periodic table?
-The general trend is that ionization energy increases as you move from left to right across the periodic table, due to the increasing effective nuclear charge attracting the electrons more strongly.
How does the ionization energy trend differ when moving down a group in the periodic table?
-When moving down a group, the ionization energy generally decreases because the valence electrons are further from the nucleus and are less strongly attracted to it.
What is the significance of the first ionization energy?
-The first ionization energy refers to the energy required to remove the first electron from a neutral atom to form a cation. It is the most commonly tested ionization energy and is a good indicator of an atom's reactivity.
Why does the ionization energy increase when moving from one ionization state to the next (e.g., from M to M+, then M+ to M2+)?
-Each subsequent ionization energy increases because it becomes more difficult to remove an electron from a cation, which already has a positive charge, compared to a neutral atom.
What are the exceptions to the ionization energy trend between groups 2A and 3A in the periodic table?
-The exceptions occur because elements in group 2A have a full s-orbital, making it more difficult to remove an electron compared to elements in group 3A, which do not have a full or half-full orbital, thus requiring less energy for ionization.
How do the ionization energies of elements in groups 5A and 6A compare, and why?
-Group 5A elements have a higher ionization energy than group 6A elements because p-orbitals in group 5A are half-full, which is a stable configuration that resists losing an electron, whereas group 6A elements have p-orbitals that are more than half-full and thus less stable.
Why is it beneficial to know the electron configurations when dealing with ionization energy exceptions?
-Knowing electron configurations helps in understanding why certain elements have higher or lower ionization energies than expected based on their group, due to the stability of half-full or full orbitals.
Can you provide an example of how to determine which element has a higher first ionization energy, given two elements from different periods?
-To determine which element has a higher first ionization energy, consider their position in the periodic table. Generally, the element higher up in a group will have a lower ionization energy, barring any exceptions. For example, comparing sodium (in period 3) and rubidium (in period 4), sodium would have a higher first ionization energy because it is higher up in the group.
Outlines
🔬 Understanding Ionization Energy and Trends
This paragraph introduces the concept of ionization energy, which is the energy required to remove an electron from an atom in its gaseous state. It explains the process of ionization, resulting in the formation of a cation, and the importance of charge balance in the reaction. The paragraph emphasizes the need to understand the trend of ionization energy across the periodic table, including the increase from left to right and up a group. It also touches on the sequential removal of electrons and the concept of first ionization energy, setting the stage for further discussion on exceptions to general trends.
📚 Ionization Energy Trends and Exceptions
The second paragraph delves deeper into the trends of ionization energy, highlighting the increase in energy required to remove an electron as you move across a period from left to right and down a group. It discusses the concept of effective nuclear charge (Z effective) and its influence on ionization energy. The paragraph also addresses exceptions to these trends, particularly for groups 2a, 5a, and 6a, where the order of ionization energy differs from the general rule. It explains the reasons behind these exceptions, such as the stability of half-full and fully filled orbitals, using beryllium and boron, as well as nitrogen and oxygen, as examples.
🔍 Comparing Ionization Energies and Exceptions
This paragraph continues the discussion on ionization energy, focusing on the comparison between elements within the same period and group, and the importance of remembering exceptions to the general trend. It provides a strategy for students to either memorize these exceptions or rely on electron configurations to solve problems. The paragraph also includes a brief mention of higher ionization energies, indicating that more energy is required to remove subsequent electrons from an atom, and it suggests that students should be prepared to answer questions about the highest ionization energy among given options.
📈 Applying Ionization Energy Trends in Practice
The final paragraph encourages students to apply their understanding of ionization energy trends and exceptions through practice. It acknowledges the complexity of chemistry and the numerous exceptions to rules, but reassures students that with practice and understanding, they can master the topic. The paragraph ends with a motivational note, urging students to study the material and re-watch the video to prepare for their exams, expressing confidence in their ability to succeed.
Mindmap
Keywords
💡Ionization Energy
💡Periodic Trend
💡Atomic Radius
💡Electron Removal
💡Cation
💡Valence Electrons
💡Ionization Energy Exceptions
💡Electron Configuration
💡Z Effective
💡Practice Problems
Highlights
Ionization energy is a key periodic trend that needs to be understood, including its exceptions.
Ionization energy is the energy required to remove an electron from an atom in its gas phase.
The ionization process results in the formation of a cation with a balance of charges.
The trend of ionization energy increases from left to right across the periodic table and decreases moving down a column.
Multiple electrons can be removed from an atom, but only one at a time, sequentially.
The first ionization energy is the focus of most tests, referring to the removal of a single electron.
Each subsequent ionization energy requires more energy to remove an electron due to increased positive charge.
The first ionization energy is the lowest, and subsequent ionization energies increase.
Z effective, or the net charge experienced by an electron, influences ionization energy.
There are exceptions to the ionization energy trend, particularly in groups 2a, 3a, 5a, and 6a.
Group 2a elements have a higher first ionization energy compared to 3a, and 5a has a higher energy than 6a.
The stability of electron configurations, especially half-full or fully filled orbitals, affects ionization energy.
Beryllium has a higher first ionization energy than boron due to the stability of a fully filled orbital.
Nitrogen requires more energy to remove an electron when it is half-full compared to oxygen.
Understanding exceptions to the ionization energy trend is crucial for solving related problems.
Practicing with ionization energy problems is essential for mastering the concept.
The video provides a comprehensive guide to understanding ionization energy and its exceptions.
Transcripts
Browse More Related Video

Periodic Trends of the Periodic Table
Ionization Energy - Basic Introduction

Electron affinity: period trend | Atomic structure and properties | AP Chemistry | Khan Academy

Trends in the Periodic Table
Periodic Table Trends: Ionization Energy

Ionization Energy Electron Affinity Atomic Radius Ionic Radii Electronegativity Metallic Character
5.0 / 5 (0 votes)
Thanks for rating: