5 Periodic Tables We Don't Use and Why
TLDRThis SciShow episode delves into the history and evolution of the periodic table, highlighting its significance in chemistry. It explains the standard long-form table's organization by groups and periods, revealing patterns in element properties. The video also explores alternative designs, from Mendeleev's predictive card arrangement to spiral and 3D representations, emphasizing the periodic table's adaptability and its role in scientific discovery and organization.
Takeaways
- 🔍 Science is fundamentally about identifying and explaining patterns, with chemistry's periodic table being a notable example.
- 📚 The periodic table is not a single, static entity; it has evolved over time with various versions proposed by scientists.
- 🧩 The standard 'long-form' periodic table is maintained by the International Union of Pure and Applied Chemistry and is highly useful for organizing elements by groups and periods.
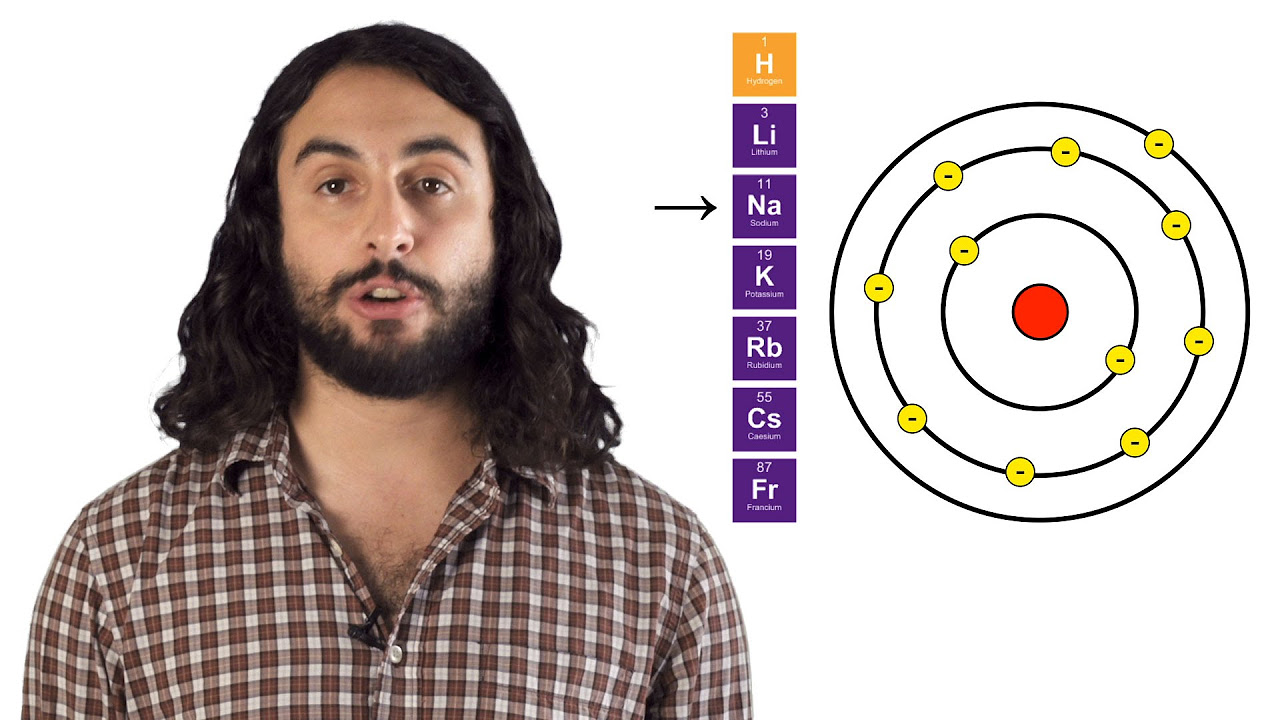
- 🌐 The arrangement of elements in the standard table reflects their electron configurations, which are key to understanding their chemical properties and relationships.
- 🔬 The atomic number, not atomic mass, is the basis for the modern periodic table, which was a significant shift from Mendeleev's original sorting method.
- 📉 Mendeleev's periodic table was revolutionary for its predictive power, allowing him to forecast the properties of undiscovered elements based on patterns.
- 🌀 The concept of a spiral periodic table, introduced by Alexandre-Émile Béguyer de Chancourtois, offers a continuous representation of elements and their properties.
- 📐 Roy Alexander's 3D design of the periodic table provides a visually connected layout that preserves groupings and offers a unique perspective on element relationships.
- 🔄 Charles Janet's left-step table, favored by physicists, illustrates the order in which electrons fill an atom's energy shells, aiding in understanding electron configurations.
- 🎨 Eric Scerri's aesthetically pleasing table, based on chemical triads, emphasizes the orderly nature of the periodic table and the predictability of element properties.
- 🛠 The periodic table is a dynamic tool that continues to be refined, reflecting ongoing scientific inquiry and the evolving understanding of elemental properties.
Q & A
What is the fundamental concept of science according to the script?
-The fundamental concept of science is about finding patterns in the world and explaining why they happen.
Why is the periodic table considered so useful in chemistry?
-The periodic table is useful because it organizes elements into groups and periods, revealing relationships and properties of elements that are crucial for understanding their chemical behavior.
Who maintains the standard periodic table and why is it important?
-The International Union of Pure and Applied Chemistry maintains the standard periodic table, which is important for standardizing how chemists work globally.
How does the arrangement of elements in the periodic table reflect their electron configurations?
-Elements in a period have a similar number of energy levels with different numbers of electrons, while elements in a group have the same number of electrons in their highest energy level (valence shell) but may have a different number of levels.
What is the significance of the valence shell in chemical bonding?
-The valence shell, which contains the outermost electrons, is significant in chemical bonding because these electrons are involved in forming chemical bonds with other elements.
Why do atoms in a group get progressively bigger as you go down the column?
-Atoms in a group get progressively bigger because each element has more energy levels than the last, with those levels located farther from the nucleus, similar to the suburbs of a city.
What is the reason behind the smaller atomic radius when moving from left to right in a period?
-The atomic radius gets smaller when moving from left to right in a period because elements have more electrons filling the same energy shells and more protons pulling on them, increasing nuclear charge.
Who is credited with the invention of the periodic table and why was 2019 declared the International Year of the Periodic Table?
-Dmitri Mendeleev is credited with the invention of the periodic table, and 2019 was declared the International Year of the Periodic Table by the UN to mark the 150th anniversary of his publication.
How did Mendeleev's original periodic table differ from the modern one?
-Mendeleev's original table was organized by atomic mass rather than atomic number, and he left spaces for elements not yet discovered, making predictions about their properties.
What was Alexandre-Émile Béguyer de Chancourtois's contribution to the periodic table?
-Alexandre-Émile Béguyer de Chancourtois constructed an early version of the periodic table known as the telluric screw, which wrapped elements around a rotating cylinder, revealing the repeating nature of elements.
What is the significance of the spiral periodic table and why might it be preferred by some?
-The spiral periodic table provides a continuous representation of elements, making it easier to see the progression of elements and their relationships, which can be beneficial for understanding periodicity.
What is unique about Roy Alexander's 3D design of the periodic table?
-Roy Alexander's 3D design folds the regular table back on itself, connecting every element to its neighbors while preserving groupings, and it was granted a patent by the US Patent Office in 1971.
Why is Charles Janet's left-step table particularly favored by physicists?
-Charles Janet's left-step table is favored by physicists because it represents the exact order in which electrons fill up an atom's available energy shells, making it a useful reference for predicting chemical and physical properties.
What is the basis for Eric Scerri's proposed new periodic table design?
-Eric Scerri's proposed new periodic table design is based on the idea of chemical triads, groups of three elements with similar properties, which can provide an elegant and orderly representation of the periodic table.
Why is the periodic table still subject to change and reinterpretation?
-The periodic table is subject to change and reinterpretation because it is a tool for organizing and understanding elements, and as new patterns and needs are discovered, the table can be modified to better serve scientific understanding.
Outlines
🔬 The Evolution of the Periodic Table
This paragraph introduces the periodic table as a fundamental tool in chemistry, highlighting its ability to reveal patterns in element properties. It mentions the standard 'long-form' periodic table, maintained by the International Union of Pure and Applied Chemistry, and its organization into groups and periods based on atomic number and electron configuration. The paragraph also touches on the historical development of the table, crediting Dmitri Mendeleev for its invention and noting the changes it has undergone since his time, including the shift from sorting by atomic mass to atomic number.
🌀 Alternative Periodic Table Designs
This section delves into various alternative designs of the periodic table, starting with Alexandre-Émile Béguyer de Chancourtois's telluric screw, a cylindrical representation that was overlooked by chemists of his time. It then discusses the 2D spiral table by Heinrich Baumhauer and its expansion by Theodor Benfey, emphasizing the continuous nature of elements in a spiral form. The paragraph also mentions Roy Alexander's 3D design, which connects every element to its neighbors while preserving useful groupings, and Charles Janet's left-step table, favored for its alignment with electron configuration patterns. Lastly, it touches on Eric Scerri's aesthetically pleasing table based on chemical triads, which were significant in the early understanding of periodicity.
🛠 The Utility and Innovation of the Periodic Table
The final paragraph reflects on the utility of the modern periodic table in conveying various patterns and information about elements, such as trends in mass, size, reactivity, and states of matter. It acknowledges the ongoing innovation and tinkering with the table's design to reveal new patterns or serve different scientific needs. The paragraph concludes by celebrating the periodic table's role in scientific understanding and the creative approaches taken to organize and interpret elemental data.
Mindmap
Keywords
💡Periodic Table
💡Atomic Number
💡Electron Configuration
💡Valence Shell
💡Energy Levels
💡Lanthanide and Actinide Series
💡Dmitri Mendeleev
💡Telluric Screw
💡Chemical Triads
💡Electron Shells
💡Charles Janet
Highlights
Science is fundamentally about identifying and explaining patterns in the world, with chemistry's periodic table being a prime example.
The periodic table is not a single, universal form but has had various versions proposed by scientists over time.
The standard or 'long-form' periodic table is maintained by the International Union of Pure and Applied Chemistry and is crucial for organizing elements into groups and periods.
The standard table's organization reveals properties and relationships between elements based on their atomic numbers and electron configurations.
Elements in a period share a similar number of energy levels, while elements in a group have the same number of valence electrons.
The periodic table's organization helps predict chemical behavior, such as the reactivity of sodium and potassium with halogens.
The size of atoms increases down a column and decreases across a period due to the number of energy levels and protons.
The periodic table's lanthanide and actinide series are placed at the bottom to fit the table on standard paper sizes.
Dmitri Mendeleev is credited with the invention of the periodic table, and 2019 was declared the International Year of the Periodic Table.
Mendeleev's table was organized by atomic mass and left spaces for undiscovered elements, allowing him to make accurate predictions about their properties.
Alexandre-Émile Béguyer de Chancourtois' telluric screw was an early attempt to visualize periodicity through a cylindrical arrangement of elements.
Heinrich Baumhauer and Theodor Benfey developed the spiral table, emphasizing the continuous nature of the periodic patterns.
Roy Alexander's 3D design of the periodic table provides a continuous and connected representation of elements, preserving useful groupings.
Charles Janet's left-step table organizes elements according to the order in which their electron shells fill, beneficial for understanding electron configurations.
Eric Scerri proposed a new table design based on chemical triads, emphasizing the aesthetic and orderly arrangement of elements.
The periodic table is a dynamic tool that continues to evolve, reflecting new patterns and serving different scientific needs.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: