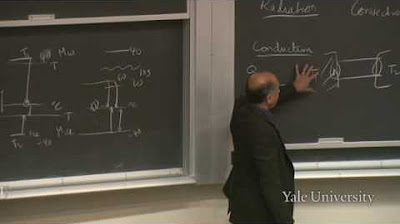Wayne Myrvold: Thermodynamics: The Basics
TLDRThis lecture delves into the fundamental concepts of thermodynamics, exploring the basic laws and principles that govern energy transfer in systems. It revisits the zeroth, first, and second laws, discussing concepts like equilibrium, heat transfer, and work. The lecturer aims to clarify misconceptions and provide a deeper understanding of thermodynamics, touching on the historical development of these ideas and their implications for modern physics.
Takeaways
- 📚 Many people either haven't taken a thermodynamics course or find the concepts fuzzy, necessitating a review of basic principles.
- 🌀 The presenter intends to clarify logical relationships in thermodynamics that are often unclear in standard textbooks.
- ⚙️ Newton's three laws of motion are fundamental, but thermodynamics has its own set of laws, including the zeroth law, which precedes the others logically.
- 🧪 The minus first law, also known as the equilibrium principle, states that an isolated system will spontaneously reach a unique equilibrium state.
- 🌡️ The zeroth law of thermodynamics establishes a transitive relation in thermal equilibrium, crucial for defining temperature scales.
- 💡 The first law of thermodynamics is essentially the conservation of energy, encompassing both kinetic and potential energy.
- 🔥 Heat flow and work are distinct modes of energy transfer; the first law differentiates between them and emphasizes that energy changes in a system are the sum of heat added and work done.
- 🚫 The second law of thermodynamics introduces the concept of entropy and states that in an isolated system, the entropy never decreases.
- 🔄 The Carnot cycle is a theoretical model used to derive the efficiency of heat engines, relating temperature scales to the efficiency of reversible processes.
- ❄️ The third law of thermodynamics posits that it's impossible to reach absolute zero in a finite number of steps, and at absolute zero, the entropy of a perfect crystal is zero.
Q & A
What is the primary topic of the presentation?
-The primary topic of the presentation is thermodynamics and its foundational concepts.
Why does the presenter discuss their experience with thermodynamics as an undergraduate?
-The presenter discusses their experience to highlight that many people either haven't taken a thermodynamics course or have forgotten much of what they learned, emphasizing the need to review basic concepts.
What is the 'minus first law' of thermodynamics according to the presentation?
-The 'minus first law' of thermodynamics, also called the equilibrium principle, states that an isolated system in an arbitrary initial state within a finite fixed volume will spontaneously attain a unique state of equilibrium.
How does the presenter define the zeroth law of thermodynamics?
-The zeroth law of thermodynamics involves the concept of thermal contact and states that if two systems are each in thermal equilibrium with a third system, they are in thermal equilibrium with each other, forming an equivalence relation for temperature.
What is the first law of thermodynamics about?
-The first law of thermodynamics, also known as the conservation of energy, states that the change in the internal energy of a system is equal to the heat added to the system minus the work done by the system.
What is a key implication of the second law of thermodynamics as presented?
-A key implication of the second law of thermodynamics is that heat cannot spontaneously flow from a colder body to a warmer body without some external work being done.
How does the Carnot cycle help in understanding the efficiency of heat engines?
-The Carnot cycle provides a theoretical model for the maximum efficiency of heat engines, showing that the efficiency depends only on the temperatures of the heat reservoirs.
What role does the concept of entropy play in thermodynamics according to the presentation?
-Entropy is a state function that quantifies the amount of thermal energy not available to do work and increases in any irreversible process, leading to the second law's statement that the entropy of an isolated system never decreases.
Why is the distinction between heat transfer and doing work important in thermodynamics?
-The distinction is crucial for defining key concepts like entropy and stating the second law of thermodynamics, which relies on understanding how energy is transferred either as heat or as work.
What is the significance of the third law of thermodynamics?
-The third law of thermodynamics states that as the temperature of a pure crystalline substance approaches absolute zero, its entropy approaches a constant minimum, providing a zero point for entropy.
Outlines
📚 Introductory Reflections on Thermodynamics Education
The speaker begins by reflecting on the audience's familiarity with thermodynamics, questioning how many have taken related courses and how well they recall the material. They admit to gaps in their own understanding from previous studies, suggesting that thermodynamics concepts can be elusive. The speaker aims to clarify logical relations better than a standard textbook, also touching on the idea that thermodynamics foundations might be less clear-cut than commonly believed.
🔄 Thermodynamics' Basic Concepts and Misconceptions
The speaker continues by discussing the basic principles of thermodynamics, highlighting the common experience of confusion during learning, especially with statistical mechanics. They introduce the concept of 'minus first law' or the equilibrium principle, which states that an isolated system will spontaneously reach a unique equilibrium state. The talk also covers the zeroth law of thermodynamics, which involves the idea of thermal contact and the transitive nature of thermal equilibrium, leading to the definition of temperature equivalence.
🔧 The First Law of Thermodynamics: Conservation of Energy
The paragraph delves into the first law of thermodynamics, which is essentially the law of conservation of energy. It discusses the concepts of kinetic and potential energy, work done on or by a system, and heat transfer. The speaker explains that energy can be transferred as work (when compressing or expanding a gas) or as heat (through thermal contact). The first law is presented in both its integral and differential forms, emphasizing the state function of internal energy and the non-state nature of heat transfer.
⚙️ The Second Law of Thermodynamics: Limitations of Energy Use
The speaker explores the second law of thermodynamics, focusing on the limitations it imposes on the use of energy. They mention Lord Kelvin's statement about the impossibility of deriving mechanical effect from a single heat source below the temperature of the coldest surrounding object. The discussion highlights the importance of temperature differences for heat engines and the concept of time asymmetry in thermodynamic processes.
⏱️ Time Asymmetry and the Second Law's Formulations
This paragraph examines the time asymmetry inherent in the second law of thermodynamics and its various formulations. The speaker discusses the Kelvin-Planck and Clausius statements, emphasizing their temporal asymmetry and the conditions under which they hold. They also touch on the historical context of these laws, including the consideration of 'inanimate material agency' and the philosophical implications of excluding intelligent agents from thermodynamic systems.
🔄 Reversible and Irreversible Processes in Thermodynamics
The speaker introduces the concepts of reversible and irreversible processes, using the Carnot cycle as an example of a reversible process. They explain that quasi-static reversible processes can be used to define state functions like entropy. The paragraph also discusses the challenges in defining heat and work in thermodynamics and the distinction between them, especially in the context of kinetic theory versus thermodynamics.
🌡️ Temperature Scales and the Connection to Ideal Gases
The speaker discusses the establishment of temperature scales, particularly the absolute or Kelvin scale, derived from the efficiency of reversible heat engines. They then connect this to the ideal gas temperature scale, which is defined by the product of pressure and volume for an ideal gas. The paragraph explores the relationship between these two temperature scales and how they can be used to define each other.
🔧 The Carnot Cycle and Its Implications for Entropy
The speaker delves into the Carnot cycle, a reversible cycle used in thermodynamics, and its implications for understanding entropy. They explain that the integral of heat over temperature around any cycle is zero, leading to the definition of entropy as a state function. The Carnot cycle's efficiency is related to the temperatures of the heat reservoirs, providing a connection between the ideal gas temperature and the absolute thermodynamic temperature.
🔍 The Foliation of State Space and the Definition of Entropy
This paragraph discusses the foliation of state space in thermodynamics, where isotherms and adiabats form a coordinate system for the state space. The speaker explains that the assumption of non-intersecting adiabats and isotherms filling the state space is crucial for defining entropy as a state function. They also touch on the implications of this assumption for the existence of a state function that remains constant along adiabatic paths.
📉 Entropy as a State Function and Its Path Independence
The speaker emphasizes that entropy is a state function, meaning its value depends only on the current state of the system, not on the path taken to reach that state. They discuss the importance of this property for entropy, especially in the context of reversible and irreversible processes. The paragraph also addresses the challenges in defining entropy and the assumptions underlying its definition.
🔧 The Second Law of Thermodynamics and Entropy
The speaker returns to the second law of thermodynamics, discussing its implications for entropy. They explain that for any process, reversible or not, the change in entropy will be non-decreasing, which is a statement of the second law. The paragraph also touches on the concept of quasi-static processes and how they relate to the state space representation of thermodynamic systems.
🔄 The Kelvin and Clausius Statements of the Second Law
The speaker explores the equivalence of the Kelvin and Clausius statements of the second law of thermodynamics. They discuss the background assumptions required for these formulations to be considered equivalent, including the positivity of absolute temperatures. The paragraph also mentions the implications of negative absolute temperatures in certain physical systems, such as spin systems with inverted populations found in lasers.
🏔️ The Third Law of Thermodynamics and Entropy at Absolute Zero
The speaker briefly touches on the third law of thermodynamics, which deals with the behavior of systems as temperature approaches absolute zero. They mention that the entropy of a system approaches a constant value, often interpreted as zero, which provides a natural zero point for entropy. The paragraph also discusses the quantum mechanical basis for the third law and its implications for substances with negative absolute temperatures.
Mindmap
Keywords
💡Thermodynamics
💡Energy Conservation
💡Heat Engines
💡Temperature
💡Entropy
💡Reversible Process
💡Carnot Cycle
💡Ideal Gas
💡State Function
💡Second Law of Thermodynamics
💡Zeroth Law of Thermodynamics
Highlights
Introduction to thermodynamics concepts for those who may not have a clear understanding from past studies.
Explanation of thermodynamics' logical relations, aiming to clarify them more than standard textbooks.
Discussion on the historical context of thermodynamics, including its development in the 19th century.
Introduction of the 'zeroth law' of thermodynamics, which is considered logically prior to Newton's laws.
Elucidation of the concept of equilibrium in thermodynamics and its significance.
Explanation of thermal contact and its importance in the zeroth law of thermodynamics.
Introduction of the first law of thermodynamics, which is essentially the conservation of energy.
Differentiation between work and heat as two distinct modes of energy transfer.
Discussion on the second law of thermodynamics, focusing on what is achievable with energy.
Introduction of the concept of quasi-static reversible processes in thermodynamics.
Explanation of the Carnot cycle and its relevance to the efficiency of heat engines.
Linking the efficiency of a reversible engine to the temperatures of two heat reservoirs.
Introduction of the concept of entropy and its role as a state function in thermodynamics.
Discussion on the third law of thermodynamics, focusing on the behavior of systems at absolute zero.
Explanation of how the third law provides a natural zero point for entropy.
Discussion on the practical applications of thermodynamics, including ideal gas thermometry.
Exploration of the relationship between ideal gas temperature and absolute temperature.
Discussion on the limitations of the ideal gas model and its approximation to real gases.
Introduction of the concept of negative absolute temperatures and their implications for thermodynamics.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: