Why Do Acids Burn?
TLDRThis script delves into the science of acids, debunking the myth of a super acid eating through the Earth. It explains chemical stability through electronegativity and bond strength, and how acids, being unstable, donate protons to seek stability. Acids' corrosive nature is attributed to their ability to disrupt protein structures, while bases cause damage by altering amino acids. The script concludes with the compatibility of acids and bases with plastic containers, highlighting electron behavior as the key to their non-reactivity.
Takeaways
- 🧪 The script begins with a fictional childhood fear of a super acid that could theoretically eat through the Earth, highlighting the imaginative nature of children.
- 🔬 Chemicals, like people, seek stability and prefer to be in a state that requires less energy, which is why they tend to react to achieve a more stable configuration.
- 🔗 The strength of chemical bonds, which determines molecular stability, is influenced by the electrostatic attraction between protons and electrons.
- 🌐 Electronegativity plays a crucial role in determining how close electrons can get to the protons in an atom, affecting the stability and reactivity of molecules.
- 🌟 Elements with higher proton-to-electron shell ratios are more electronegative, attracting electrons more strongly and influencing their reactivity.
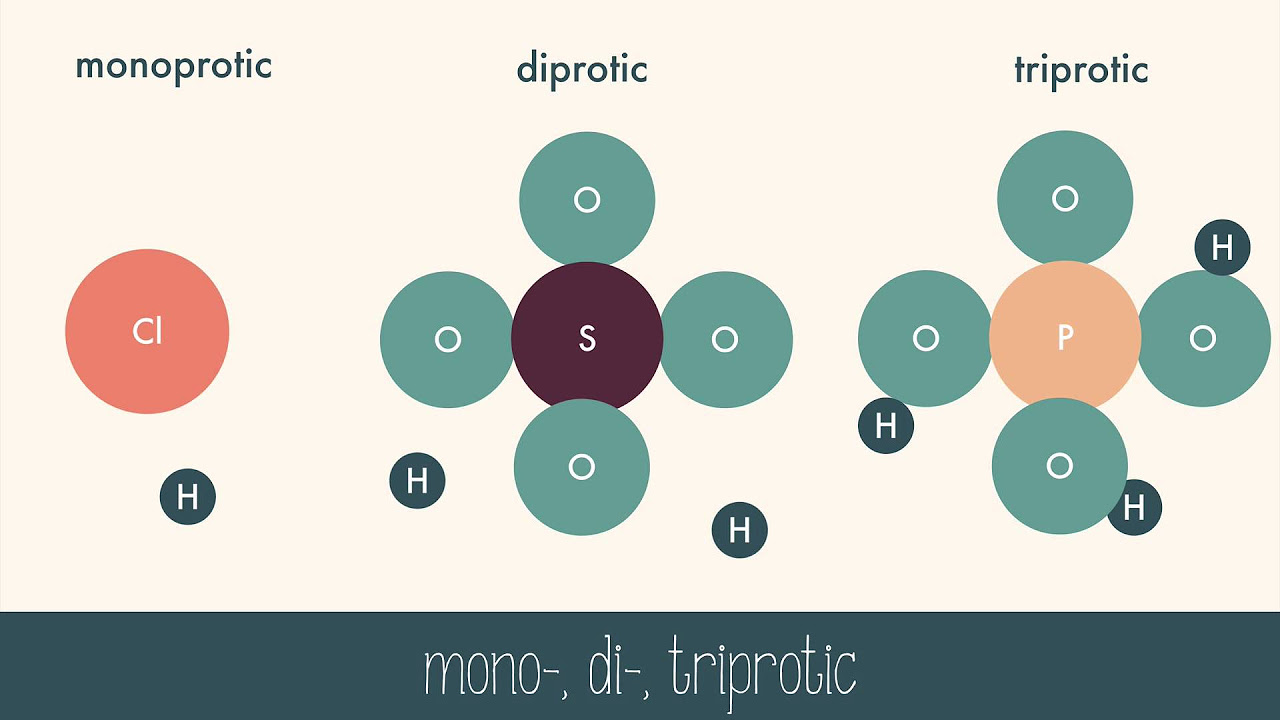
- 🍋 Acids are described according to the Bronsted-Lowry definition as molecules that can donate a proton, and their instability drives them to react with other substances.
- 🔝 Acids are often unstable due to the presence of a hydrogen atom bonded to an electronegative atom, creating a weak bond that seeks to be stabilized.
- ⚗️ Acids constantly seek to offload their extra hydrogen to lone electron pairs in other molecules, which is the basis for their reactivity and corrosive nature.
- 🔥 Acids can cause chemical and thermal burns by disrupting the structure of proteins, which are vital for life due to their precise folding and function.
- 🧬 The integrity of proteins is maintained by intermolecular forces like salt bridges and hydrogen bonds, which can be compromised by acids and bases.
- 🧴 Bases can also disrupt proteins, but through a different mechanism involving the removal of a proton from the amine ion and subsequent reactions.
- 🛢️ Plastic containers, such as polyethylene, can safely store caustic substances because the bonds within the plastic are not easily broken by acids or bases without significant energy input.
Q & A
What is the main fear described in the script from the narrator's childhood?
-The main fear described is that a mad scientist accidentally spills super acid in a laboratory, which then begins to eat through the Earth.
Why is the concept of a super acid eating through the Earth considered impossible?
-It's considered impossible because the chemical world seeks stability, and substances tend to react to achieve a more stable state rather than cause destruction.
What is the reason behind the stability of molecules in the chemical world?
-The stability of molecules relies on the strength or energy within the bonds holding them together, which are formed from electrostatic attraction between protons and electrons.
What is electronegativity and how does it affect the stability of elements?
-Electronegativity is the ratio between protons in the nucleus and the electron shells underneath the valence shell. It affects stability by influencing how close electrons can be to the protons, with elements having higher proton-to-electron ratios being more stable.
According to the Bronsted-Lowry definition, what is an acid?
-An acid, according to the Bronsted-Lowry definition, is a molecule that can donate a proton or hydrogen atom.
Why are acids considered unstable molecules?
-Acids are unstable because they have a hydrogen atom bonded to an electronegative atom, creating a bond that is not very strong, and they are always looking for a lone electron pair to stabilize themselves by donating their hydrogen.
How do acids cause a chemical burn when they come into contact with our skin?
-Acids cause a chemical burn by reacting with the carboxylate ions in proteins, causing them to receive a proton and lose their negative charge, which can lead to the unfolding and malfunctioning of proteins.
What is the difference between how acids and bases cause damage to proteins?
-Acids donate a proton to the carboxylate ion, neutralizing its charge, while bases can rip a proton from the amine ion and then use the formed water to facilitate alkaline hydrolysis, splitting other organic bonds.
Why can't acids and bases eat through simple plastic containers?
-Acids and bases can't eat through simple plastic like polyethylene because there are no free electrons available for the acids to donate protons or for the bases to accept protons, resulting in no reaction.
What is the role of intermolecular forces in maintaining the shape of proteins?
-Intermolecular forces, such as salt bridges and hydrogen bonds, play a crucial role in maintaining the precise folding of proteins, which is essential for their function.
Why is a strong base theoretically a faster option for dissolving body components compared to an acid?
-A strong base is faster because it can cause alkaline hydrolysis, breaking down organic bonds more extensively and quickly than an acid, which primarily neutralizes charges on specific sites.
Outlines
🧪 Understanding Chemical Stability and Acids
This paragraph explores the concept of chemical stability, using the childhood fear of a 'super acid' as a metaphor. It explains that in the chemical world, substances seek stability, similar to how humans prefer ease. Stability in molecules is determined by the strength of the bonds, which are influenced by electronegativity—the ratio of protons to electron shells. Acids are described as unstable molecules that donate protons, seeking to stabilize themselves by reacting with other molecules. The paragraph also touches on the structure of acids, often having a hydrogen atom bonded to an electronegative atom, which creates a weak bond and contributes to their instability.
🔬 The Effects of Acids and Bases on Proteins and Storage of Caustic Substances
The second paragraph delves into the impact of acids and bases on proteins, which are vital for life due to their precise folding maintained by intermolecular forces like salt bridges and hydrogen bonds. Acids can disrupt this by donating protons to carboxylate ions, causing proteins to unfold and lose function. Bases, on the other hand, can rip protons from amine ions, leading to similar destructive effects. The paragraph also discusses the storage of these caustic substances, explaining that simple plastics like polyethylene are resistant to acids and bases due to the lack of suitable bonds for reaction, thus preventing the substances from 'eating through' the container.
Mindmap
Keywords
💡Stability
💡Electronegativity
💡Acid
💡Proton
💡Electron
💡Chemical Bond
💡Corrosiveness
💡Protein
💡Hydrogen Bond
💡Base
💡Polyethylene
Highlights
The fear of a super acid eating through the Earth is debunked as scientifically impossible.
Chemicals, like humans, seek stability and prefer to be in a state that requires less energy.
Molecular stability is determined by the strength of bonds formed by electrostatic attraction between protons and electrons.
Electronegativity plays a crucial role in dictating how close electrons can be to the atom's nucleus.
Larger atoms with more electrons experience electron repulsion, affecting their stability.
Electronegativity can be conceptualized as the ratio of protons to electron shells, influencing electron behavior.
Acids are described by the Bronsted-Lowry definition as molecules that can donate a proton.
Acids are naturally unstable molecules, seeking to stabilize themselves by donating protons.
The instability of acids is often due to a hydrogen atom bonded to an electronegative atom.
Acids constantly seek to offload their extra hydrogen to lone electron pairs to stabilize themselves.
Strong acids are very unstable and can force almost any molecule to accept their hydrogen.
Acids and bases can cause chemical and thermal burns by destroying proteins, essential for life.
Proteins rely on precise folding and intermolecular forces, such as salt bridges and hydrogen bonds, for function.
Acids can cause proteins to unfold by donating protons to carboxylate ions, disrupting their structure.
Bases can also disrupt proteins but through a different mechanism involving the amine ion.
Strong bases can dissolve body components faster than acids due to their ability to initiate alkaline hydrolysis.
Plastic containers can safely store caustic substances because the electrons in the plastic are not reactive with acids or bases.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: