Acidity and Basicity of Alcohols
TLDRThis video from Leah4Sci explores the amphiprotic nature of alcohols, their ability to act as both acids and bases. It delves into the PKA values, comparing ethanol's weak acidity to ethanoic acid's stronger acidity. The tutorial explains how alcohols form oxonium ions upon protonation and alkoxides when deprotonated, highlighting the stability and reactivity of these species. It also covers the formation of alkoxides using strong bases and the CARIO principles to rank acid-base strength without memorizing PKA values, providing a foundational understanding of alcohols' behavior in organic chemistry.
Takeaways
- 📚 Alcohols are amphiprotic, meaning they can act as both acids and bases.
- 🔬 Ethanol can be a proton donor (acid) or a proton acceptor (base).
- ⚖️ The equilibrium favors the formation of the conjugate acid (oxonium) over the conjugate base (alkoxide).
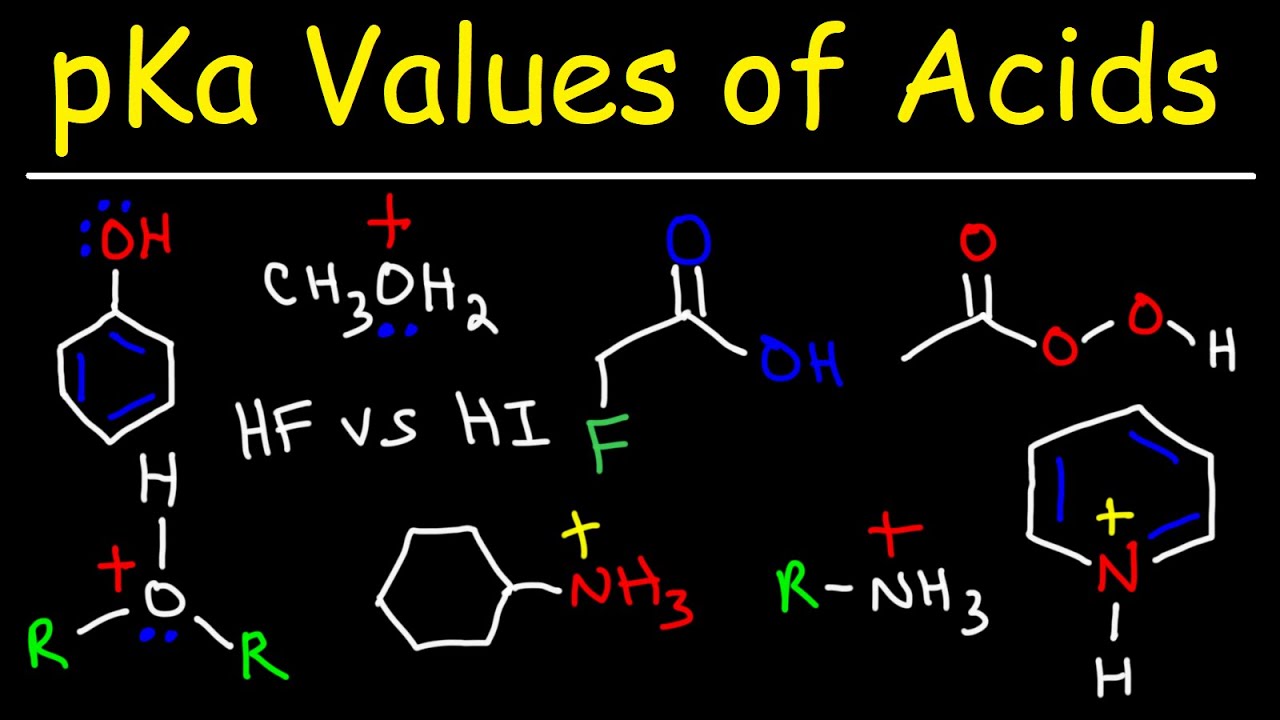
- 📊 The pKa of ethanol is 15.9, indicating it's a relatively weak acid compared to stronger acids like ethanoic acid with a pKa of 4.8.
- 🧪 Alcohols can be deprotonated to form alkoxides using strong bases like sodium hydride or metals like sodium.
- 🔄 Sodium hydride reactions are irreversible due to the formation of H2 gas.
- 🧠 Understanding acidity and basicity involves CARIO principles: Charge, Atom, Resonance, Induction, and Orbital Hybridization.
- 💡 Resonance stabilization can increase acidity, as seen in enols compared to ethanol.
- 🔍 Inductive effects, like the presence of chlorine, can also increase the acidity of alcohols.
- 📈 Alcohols with higher electronegativity atoms or stabilizing groups are more acidic.
Q & A
What is the definition of an amphiprotic molecule?
-An amphiprotic molecule is one that can act as both an acid and a base, meaning it can be a proton donor or a proton acceptor.
Why is the term 'amphiprotic' used to describe alcohols?
-The term 'amphiprotic' is used for alcohols because they can act as both acids and bases due to their ability to donate or accept protons.
What is the difference between an alcohol acting as an acid and as a base?
-When an alcohol acts as an acid, it donates a proton, forming an oxygen with three lone pairs and a negative charge. When it acts as a base, it accepts a proton, forming a protonated oxygen with a positive charge.
What is the preferred reaction for alcohols, acting as an acid or a base?
-The preferred reaction for alcohols is to act as a base, as the resulting protonated alcohol (oxonium) is relatively stable, whereas the alkoxide formed when acting as an acid is less stable and more reactive.
What is the PKA value of ethanol, and what does it indicate about its acidity?
-The PKA value of ethanol is 15.9, which indicates that it is a relatively weak acid because a higher PKA value corresponds to a weaker acid.
How does the PKA value of ethanoic acid compare to that of ethanol, and what does this mean for their relative acidity?
-Ethanoic acid has a PKA of 4.8, which is lower than that of ethanol. This means that ethanoic acid is a stronger acid than ethanol, as a lower PKA indicates greater acidity.
What is an oxonium ion, and how is it related to alcohols in reactions?
-An oxonium ion is a positively charged oxygen atom that results when an alcohol picks up a proton. It is commonly seen in many organic chemistry reactions, such as SN1 reactions, where it is formed by the reaction of an alcohol with an acid catalyst.
What is the difference between the reactions of alcohol with sodium hydride and sodium metal?
-In the reaction with sodium hydride, sodium acts as a spectator ion, and the hydride ion acts as a strong base to deprotonate the alcohol, forming an alkoxide and hydrogen gas. In contrast, with sodium metal, the sodium atom donates an electron to the hydrogen of the alcohol, leading to the formation of an alkoxide and hydrogen gas through a radical reaction.
What is an alkoxide anion, and how is it formed?
-An alkoxide anion is a deprotonated alcohol, characterized by an oxygen atom with three lone pairs and a negative charge. It can be formed by reacting the alcohol with a strong base like sodium hydride or by reacting with a neutral metal like sodium.
What are the CARIO principles, and how are they used to rank the strength of acids and bases?
-CARIO stands for Charge of the atom, the Atom being compared, Resonance, Induction, and Orbital Hybridization. These principles are used to rank the strength of acids and bases by considering the charge state, the type of atom, the resonance stabilization, the inductive effect, and the hybridization of the atom involved in the acid-base reaction.
How does the presence of a chlorine atom on the carbon atom in ethanol affect its acidity?
-The presence of a chlorine atom, which is highly electronegative, pulls electron density towards itself from the carbon atom, making the adjacent carbon partially positive. This inductive effect makes the oxygen atom less burdened with the negative charge when deprotonated, resulting in a more stable conjugate base and thus a stronger acid.
What is the significance of the PKA values in comparing the acidity of ethanol and chloroethanol?
-The PKA values indicate the relative acidity of the alcohols. Ethanol has a PKA of about 16, while chloroethanol has a PKA of about 14. The lower PKA of chloroethanol means it is almost a hundred times stronger as an acid compared to ethanol.
Why is the phenol mentioned in the context of alcohols and acidity?
-Phenol is mentioned because it is a specific type of alcohol where resonance significantly impacts its acidity. The resonance stabilization of the phenoxide ion, the conjugate base of phenol, makes phenol more acidic than other alcohols.
Outlines
🍸 Amphiprotic Nature of Alcohols and Their Acid-Base Reactions
This paragraph introduces the concept of amphiprotic molecules, focusing on alcohols like ethanol. It explains how alcohols can act as both acids and bases, depending on whether they donate or accept protons. The paragraph also discusses the stability of the resulting molecules, such as the oxonium ion and alkoxide ion, and how this relates to the equilibrium of acid-base reactions. The PKA values of ethanol and ethanoic acid are compared to illustrate the relative strength of these acids, with ethanol being a weaker acid due to its higher PKA value.
🔬 Formation of Alkoxide Ions and the Role of Alcohols in Organic Reactions
This section delves into the formation of alkoxide ions through the deprotonation of alcohols using strong bases like sodium hydride or neutral metals like sodium. It highlights the irreversibility of these reactions due to the escape of hydrogen gas and the formation of stable alkoxide ions. The paragraph also explains how alcohols can act as solvents and participants in reactions like SN1, where they form oxonium ions that are important in organic chemistry.
🌐 Factors Influencing the Acidity and Basicity of Alcohols
The third paragraph explores the factors that affect the acidity and basicity of alcohols, using the CARIO principles (Charge of the atom, Atom being compared, Resonance, Induction, and Orbital Hybridization). It discusses how electronegativity and the ability of atoms to stabilize negative charges influence their acidity. The role of resonance in stabilizing the negative charge in enols and enolates is also covered, as well as the impact of inductive effects from electronegative atoms like chlorine on acidity.
📚 Conclusion and Further Exploration of Alcohols
The final paragraph wraps up the discussion on the acidity and basicity of alcohols, encouraging viewers to apply the understanding gained from the video to other areas of chemistry. It mentions the upcoming video on phenols and their unique acidity due to resonance, and invites viewers to visit Leah4Sci's website for more resources on alcohols, including a practice quiz and cheat sheet.
Mindmap
Keywords
💡Amphiprotic
💡Oxonium Ion
💡Alkoxide
💡Acid/Base Equilibrium
💡PKA
💡Conjugate Acid/Base
💡Electronegativity
💡Resonance
💡Inductive Effect
💡Hybridization
💡CARIO Principles
Highlights
Alcohols are amphiprotic, meaning they can act as both acids and bases by being proton donors or acceptors.
Ethanol serves as an example to explain the concepts of protonation and deprotonation in alcohols.
The stability of oxonium ions and alkoxide ions determines the equilibrium position in acid-base reactions of alcohols.
Ethanol's PKA value of 15.9 indicates it is a relatively weak acid compared to stronger acids like ethanoic acid with a PKA of 4.8.
Oxonium ions are common in many organic chemistry reactions, such as SN1 reactions.
Alcohols are more likely to be deprotonated to form a conjugate acid than to form a conjugate base due to the strength of the resulting bases.
Alkoxide anions are formed by reacting alcohols with strong bases like sodium hydride, making the reaction irreversible.
Neutral metal reactions with alcohols, such as with sodium, can also lead to the formation of alkoxide ions.
Alkoxides such as methoxide, ethoxide, and tert-butoxide are important nucleophiles and bases in substitution and elimination reactions.
Understanding the CARIO principles helps in ranking the strength of acids and bases without memorizing PKA values.
Charge of the atom plays a significant role in determining the acidity or basicity of a molecule.
Electronegativity and the atom's ability to stabilize a negative charge are key factors in acid-base chemistry.
Resonance stabilization of the conjugate base can increase the acidity of a molecule, as seen with enols.
The inductive effect caused by electronegative atoms like chlorine can influence the acidity of alcohols.
Orbital hybridization, though less common in alcohols, affects the acidity, as seen in phenols.
The video provides a comprehensive guide on the acid-base chemistry of alcohols with practical examples and theoretical insights.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: