[H2 Chemistry] 2023 Topic 2 Chemical Bonding 3
TLDRIn this third lecture, we focus on the Valence Shell Electron Pair Repulsion (VSEPR) theory to understand the shapes of molecules and polyatomic ions. Section 6 introduces key molecular geometries such as linear, trigonal planar, tetrahedral, trigonal pyramidal, bent, and octahedral. The lecture also explains the influence of lone pairs on bond angles and shapes. Section 7 covers molecular polarities, emphasizing the impact of electronegativity differences and molecular shapes on dipole moments. The lecture concludes with exercises to predict molecular shapes and polarities using VSEPR theory.
Takeaways
- 📚 The lecture focuses on the Valence Shell Electron Pair Repulsion (VSEPR) theory, which predicts the shapes of molecules and polyatomic ions based on electron group repulsion.
- 🔍 Students are required to know the shapes and bond angles of specific molecules like BF3 (trigonal planar), CO2 (linear), CH4 (tetrahedral), and others, using VSEPR theory.
- 🧩 The importance of distinguishing between bond pairs and lone pairs in molecules is emphasized for understanding molecular geometry.
- 📐 VSEPR theory is based on principles such as electron groups arranging themselves to be as far apart as possible to minimize repulsion, with lone pairs exerting more repulsion than bonding pairs.
- 📝 The lecture introduces the concept of hybridization of atomic orbitals, which is crucial for explaining the bonding and geometry in molecules like methane (sp3 hybridization) and ethene (sp2 hybridization).
- 🔬 The polarity of bonds and molecules is discussed, with polar covalent bonds forming due to differences in electronegativity between atoms, leading to a partial charge distribution.
- ⚛️ The concept of ionic bonding with covalent character is introduced, where the anion's electron cloud can be polarized by the cation, leading to a partial covalent bond character.
- 🌐 The overall polarity of a molecule is determined by both the presence of polar bonds and the molecular geometry, which dictates whether the bond dipoles cancel out or result in a net dipole moment.
- 📉 The polarity of molecules is illustrated with examples, showing how molecules with polar bonds can be non-polar if their geometry allows for the cancellation of bond dipoles, such as in CO2 and CCl4.
- 📚 The lecture concludes with exercises to apply the concepts learned, including predicting the shape and polarity of various molecules and understanding the implications of hybridization on bond strength.
Q & A
What are the two main sections discussed in the third lecture?
-The third lecture primarily focuses on Section 6, which discusses the shapes of molecules and polyatomic ions, and Section 7, which covers the concept of polarities in chemistry.
What is the Valence Shell Electron Pair Repulsion (VSEPR) theory?
-The VSEPR theory is a model used in chemistry to predict the shapes and bond angles of molecules and polyatomic ions based on the repulsion between electron pairs in the valence shell of the central atom.
What are the basic shapes of molecules that students need to know according to the syllabus?
-The five basic shapes that students need to know are linear, trigonal planar, tetrahedral, trigonal pyramidal, and octahedral.
How does the VSEPR theory account for the shape of a molecule with lone pairs?
-The VSEPR theory accounts for the shape of a molecule with lone pairs by considering the electron groups around the central atom, including both bonding electron pairs and lone pairs. The shape described does not include the lone pairs, but their presence affects the actual molecular shape.
What is the difference between bond pairs and lone pairs in the context of VSEPR theory?
-Bond pairs are electrons that are involved in bonding between atoms, while lone pairs are electrons that are not involved in any form of bonding and are attracted by only one nucleus.
Why is the term 'electron groups' preferred over 'electron pairs' in VSEPR theory?
-The term 'electron groups' is preferred because it more accurately represents the number of electrons around the central atom, whether they are involved in single, double, or triple bonds, which are all considered as one electron group in the context of VSEPR theory.
What is the significance of the bond angle in determining the polarity of a molecule?
-The bond angle is significant in determining the polarity of a molecule because it affects how the individual bond dipoles are oriented in space. If the bond dipoles cancel each other out, the molecule is non-polar; if they do not, the molecule is polar.
How does the electronegativity of atoms affect the polarity of a covalent bond?
-The electronegativity of atoms affects the polarity of a covalent bond by causing a difference in the attraction between the atoms for the shared electrons. If the electronegativity difference is significant, the bond is polar, with a partial positive charge on the less electronegative atom and a partial negative charge on the more electronegative atom.
What is the relationship between the hybridization state of an atom and the strength of the covalent bonds it forms?
-The hybridization state of an atom affects the strength of the covalent bonds it forms because hybrid orbitals with a greater percentage of s character result in electron density being closer to the nucleus, leading to stronger bonds.
How does the concept of polarizability relate to the difference between ionic and covalent bonds?
-Polarizability is the ability of an anion's electron cloud to be distorted by a nearby cation. In ionic bonds with significant covalent character, the anion is highly polarizable, and the electron cloud can be distorted towards the cation, leading to partial covalent character in what is otherwise considered an ionic bond.
Outlines
📚 Introduction to Lecture 3 and Molecular Shapes
The lecture begins with an introduction to the third session, focusing on sections six and seven. Section six discusses the shapes of molecules and polyatomic ions, introducing the Valence Shell Electron Pair Repulsion (VSEPR) theory. This theory is fundamental for understanding molecular geometry. Section seven briefly covers polarities. The lecture aims to challenge students with these concepts and emphasizes the importance of knowing the shapes and bond angles of specific molecules like BF3, CO2, CH4, ammonia, H2, and F6. The VSEPR theory is the central tool for predicting molecular shapes and bond angles, considering electron groups around the central atom.
🔬 Understanding Electron Pairs and VSEPR Theory
The paragraph delves deeper into the VSEPR theory, explaining the concept of electron groups or domains around central atoms in molecules or ions. These electron groups repel each other and arrange themselves to be as far apart as possible to minimize repulsion. The theory's first principle is that electron groups will spread out to minimize repulsion. The second principle highlights that repulsion between lone pairs of electrons is greater than between lone pairs and bonding pairs, and between bonding pairs themselves. Understanding these principles is crucial for predicting molecular shapes and is a significant part of the lecture's content.
📐 Basic Molecular Shapes and Electron Group Arrangements
This section discusses the five basic molecular shapes that students need to know: linear, trigonal planar, tetrahedral, trigonal pyramidal, and octahedral. It explains how electron groups arrange themselves around a central atom to form these shapes, with specific angles between them that minimize repulsion. The paragraph provides a step-by-step explanation of how two, three, four, five, and six electron groups would arrange themselves, leading to the respective molecular shapes. It also touches on the concept of electronegativity and how it affects the bond angles in molecules like water (H2O) and hydrogen sulfide (H2S).
🧩 Advanced Molecular Shapes and the Impact of Lone Pairs
Building on the basic shapes, this paragraph explores more complex molecular geometries that result from the presence of lone pairs of electrons. It explains how the inclusion of lone pairs affects the overall shape of molecules, using examples like methane (CH4) with a tetrahedral electron group arrangement but a trigonal pyramidal molecular shape due to one lone pair. The paragraph also discusses how the position of lone pairs (axial or equatorial) influences the shape and stability of molecules, with a focus on minimizing electronic repulsion.
🤔 Misconceptions and the Importance of Understanding VSEPR Theory
The speaker addresses common misconceptions about the VSEPR theory, emphasizing that it should not be memorized but understood. They explain that by grasping the principles of electron group arrangement and repulsion, students can predict molecular shapes more effectively. The paragraph also introduces the concept of Lewis structures, which include the representation of lone pairs and bonding electrons, and their importance in visualizing molecular geometry.
🔍 Detailed Analysis of Bond Angles and Electron Pair Repulsion
This section provides a detailed examination of bond angles in molecules with different electron group arrangements. It explains how the presence of lone pairs affects bond angles, using examples such as ammonia (NH3) and water (H2O) to illustrate how bond angles deviate from the ideal tetrahedral angle of 109.5 degrees. The paragraph reinforces the idea that understanding electron pair repulsion is key to predicting bond angles and molecular shapes accurately.
🏷️ Recap of VSEPR Theory Principles and Molecular Shape Prediction
The lecturer recaps the principles of the VSEPR theory and how to apply them to predict molecular shapes. They emphasize the importance of considering the number of electron groups and lone pairs around the central atom to determine the shape. The paragraph also discusses the significance of electronegativity in the context of bond angles and provides examples of molecules like PCl3 and CS2 to illustrate the concepts discussed in the lecture.
🧠 Understanding Molecular Symmetry and the Formation of Covalent Bonds
This paragraph focuses on the concept of molecular symmetry and the formation of covalent bonds. It explains the preference for symmetrical structures in molecules and the avoidance of dative covalent bonds unless necessary. The speaker discusses the importance of minimizing formal charges and the role of electronegativity in determining the most stable molecular structure, using examples like CS2 and its possible structural arrangements.
📘 Application of VSEPR Theory to Complex Molecules and Ions
The speaker applies the VSEPR theory to more complex molecules and ions, such as Xenon difluoride (XeF2), PCl5, and PCl6-. They demonstrate how to determine the number of electron groups, the arrangement of lone pairs, and the resulting molecular shapes. The paragraph also covers the prediction of bond angles and the impact of negative charges on molecular geometry, providing a comprehensive application of the VSEPR theory to a variety of chemical species.
🔬 Further Exploration of Molecular Geometry and Hybridization
This section delves into the hybridization of atomic orbitals, a concept closely related to the VSEPR theory. It discusses the need for hybridization to explain the observed shapes and bond angles in molecules that cannot be accounted for by the simple VSEPR model. The paragraph introduces the idea of hybrid orbitals, such as sp3, sp2, and sp, and how they relate to the formation of sigma and pi bonds in molecules like ethane (C2H6), ethene (C2H4), and acetylene (C2H2).
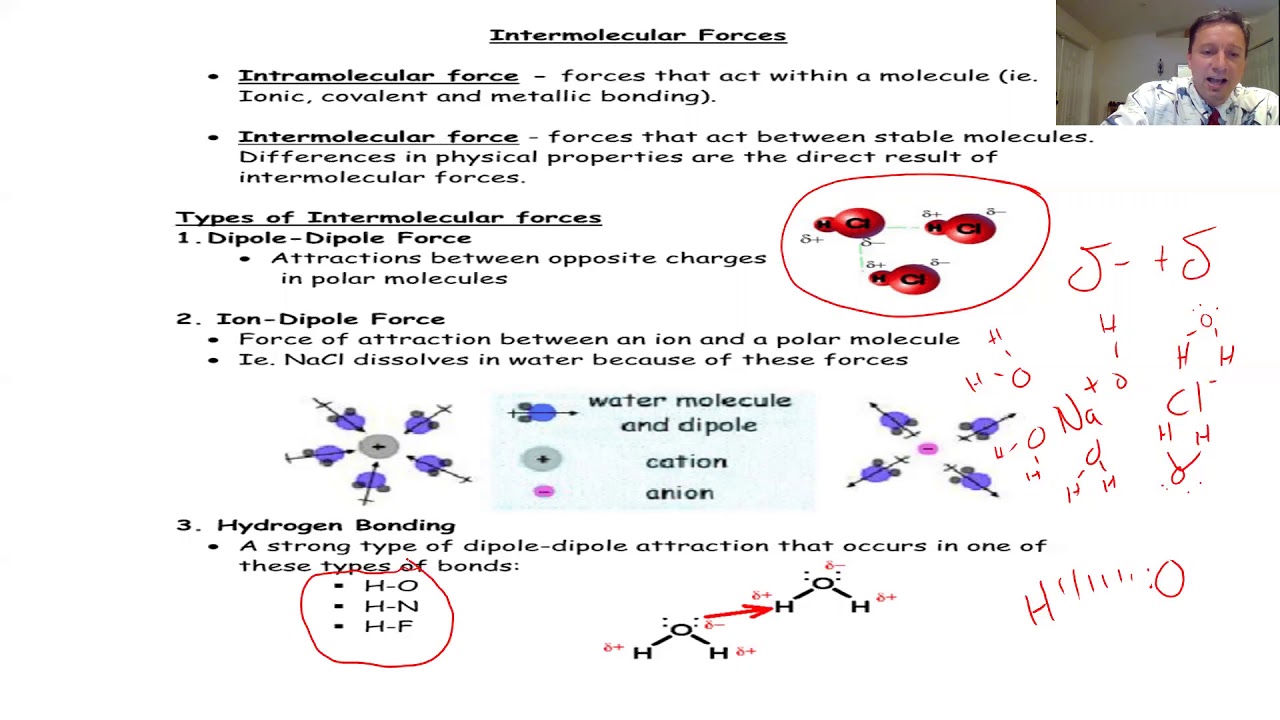
📚 Conclusion of Lecture 3 and Preview of Intermolecular Forces
The lecture concludes with a summary of the key points covered in lecture three, including the VSEPR theory, molecular shapes, bond angles, and hybridization of atomic orbitals. The speaker also provides a preview of lecture four, which will focus on intermolecular forces of attraction. This sets the stage for further exploration of physical chemistry concepts that govern the behavior of molecules in different states of matter.
Mindmap
Keywords
💡VSEPR Theory
💡Electron Groups
💡Molecular Geometry
💡Lone Pairs
💡Bond Angles
💡Polar Covalent Bonds
💡Nonpolar Covalent Bonds
💡Polar Molecules
💡Nonpolar Molecules
💡Hybridization
💡Pi Bonds
Highlights
Introduction to Valence Shell Electron Pair Repulsion (VSEPR) theory for predicting molecular shapes.
Explanation of different molecular shapes such as trigonal planar, linear, tetrahedral, trigonal pyramidal, bent, and octahedral.
Discussion on how to use VSEPR theory to predict bond angles and molecular shapes based on electron groups.
Importance of understanding the difference between bond pairs and lone pairs in VSEPR theory.
Explanation of electron domains and how they determine molecular geometry.
Illustration of how lone pair repulsion is more significant than bond pair repulsion in determining molecular shape.
Examples of predicting shapes and bond angles for molecules like BF3, CO2, CH4, NH3, and H2O.
Introduction to hybridization theory, including sp3, sp2, and sp hybridization.
Discussion on the significance of hybrid orbitals in forming molecular bonds and determining molecular geometry.
Explanation of how sp3 hybridization leads to a tetrahedral shape in molecules like methane.
Description of how sp2 hybridization leads to a trigonal planar shape in molecules like ethene.
Explanation of how sp hybridization leads to a linear shape in molecules like acetylene.
Importance of bond polarity and how electronegativity differences lead to polar and non-polar covalent bonds.
Discussion on how molecular shape affects the polarity of molecules, with examples of polar and non-polar molecules.
Exercises on predicting molecular shapes and bond angles using VSEPR theory and hybridization concepts.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: