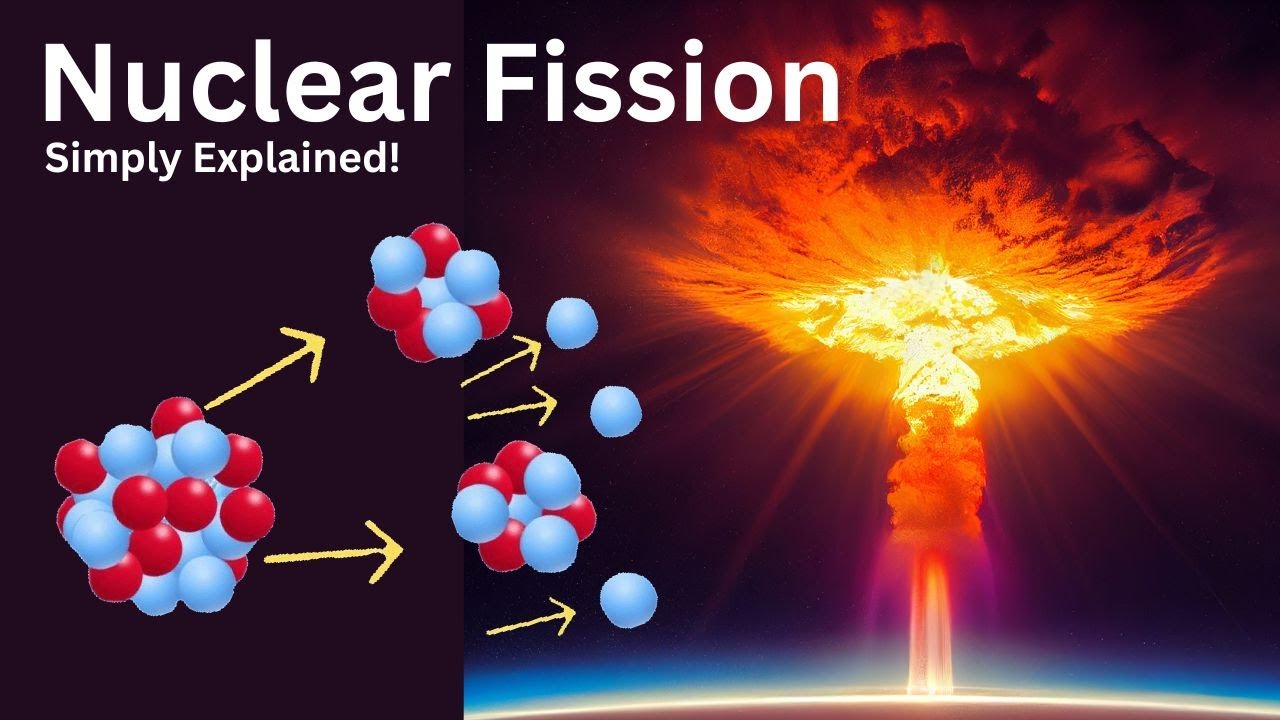
Difference between Nuclear Fission and Nuclear Fusion
TLDRThis video from MooMoo Math and Science explains the fundamental differences between nuclear fission and fusion. Nuclear fission involves splitting heavy atoms like uranium-235 into smaller elements, releasing energy, neutrons, and radioactive byproducts. It powers 439 reactors worldwide, used for electricity generation. In contrast, nuclear fusion merges light elements like hydrogen isotopes to form helium, releasing vast energy without radioactive waste. It's the process that powers stars and requires extreme temperatures for initiation on Earth. The video concludes with a reminder to spread kindness.
Takeaways
- 🔬 Nuclear fission is the process of splitting a large atom, such as uranium-235, into smaller elements.
- 💥 The splitting of uranium-235 results in the formation of unstable uranium-236, which quickly splits and releases neutrons, gamma rays, and energy.
- ⛓️ The release of additional neutrons can cause a chain reaction, where these neutrons strike other uranium-235 atoms, repeating the process.
- 🔥 Nuclear fission is a dense source of energy, but it is less than that of nuclear fusion.
- ☢️ Nuclear fission produces radioactive byproducts such as iodine-131, cesium-137, and strontium-90, which can be hazardous to health and the environment.
- 🌡️ The heat generated from nuclear fission can be used to heat steam, which then turns a turbine to produce electricity.
- 🌐 There are currently 439 nuclear reactors in operation across 30 countries worldwide.
- 🔬 Nuclear fusion involves combining lighter elements, such as isotopes of hydrogen (deuterium and tritium), to form a larger element like helium.
- ⚡️ Nuclear fusion releases a significant amount of energy, more than nuclear fission, and does not produce radioactive byproducts.
- 🌟 Nuclear fusion is the energy source of stars, initiated when the core of a protostar becomes hot enough to start the fusion process.
- 🌡️ On Earth, a temperature of 100 million degrees Celsius is estimated to be needed to initiate hydrogen fusion into helium.
Q & A
What is nuclear fission?
-Nuclear fission is the process of splitting a large atom, such as uranium-235, into smaller elements. It starts with a neutron striking uranium-235, leading to the formation of unstable uranium-236, which then splits into lighter elements, releasing additional neutrons, gamma rays, and energy.
What happens during the nuclear fission of uranium-235?
-When uranium-235 undergoes nuclear fission, it is struck by a neutron, turning into unstable uranium-236. This then splits into lighter elements, releasing more neutrons, gamma rays, and energy. This process can trigger a chain reaction.
How is nuclear fission different from nuclear fusion?
-Nuclear fission involves the splitting of a large atom into smaller elements, whereas nuclear fusion is the process of combining two or more lighter elements to form a heavier one. Fusion requires extremely high temperatures and is the energy source of stars.
What are the energy outputs of nuclear fission and fusion?
-Nuclear fission releases a significant amount of energy, much greater than a chemical reaction, but it is less than the energy produced by nuclear fusion. Fusion creates more energy than fission and does not create radioactive byproducts.
What are some radioactive byproducts created by nuclear fission?
-Some radioactive byproducts of nuclear fission include iodine-131, cesium-137 (ccm-137 in the transcript seems to be a typo), and strontium-90. These can remain radioactive for thousands of years and exposure can cause sickness, cancer, and even death.
How is the heat from nuclear fission utilized to produce electricity?
-The heat generated from nuclear fission is used to heat steam, which then turns a turbine. The spinning turbine drives a generator to produce electricity.
How many nuclear reactors are currently in operation worldwide?
-As of the information in the script, there are 439 nuclear reactors in operation in 30 countries around the world.
What are the basic elements involved in nuclear fusion?
-Nuclear fusion involves the fusing of two or more lighter elements into a larger one. For example, two isotopes of hydrogen, deuterium and tritium, can fuse to produce helium and release a neutron along with a large amount of energy.
What is a protostar and how does it relate to nuclear fusion?
-A protostar is a very young star. The energy released from the collapse of gas into a protostar causes its core to become extremely hot. When the core reaches a temperature of around 100 million degrees Celsius, nuclear fusion begins, which is the process that powers stars.
What is the temperature required on Earth to initiate hydrogen fusion into helium?
-It is estimated that a temperature of 100 million degrees Celsius is needed on Earth to initiate the fusion of hydrogen into helium.
What is the main advantage of nuclear fusion over nuclear fission in terms of environmental impact?
-Nuclear fusion has the advantage of not creating radioactive byproducts, unlike nuclear fission. This makes fusion a cleaner energy source in terms of long-term environmental impact.
Outlines
🔬 Nuclear Fission and Fusion Basics
This paragraph introduces the fundamental concepts of nuclear fission and fusion. Nuclear fission involves splitting a large atom like uranium-235 into smaller elements, triggered by a neutron strike, which creates an unstable uranium-236 that subsequently splits, releasing neutrons, gamma rays, and energy. This process can initiate a chain reaction. The energy produced is denser than that of chemical reactions but less than fusion. Byproducts of fission, such as iodine-131, cesium-137, and strontium-90, can be radioactive and harmful. Fission's heat can be harnessed to generate electricity, with 439 nuclear reactors currently operational worldwide. In contrast, nuclear fusion is the merging of lighter elements, like hydrogen isotopes deuterium and tritium, to form a heavier element like helium, releasing a neutron and significant energy. Fusion requires extreme temperatures, like those found in stars, and does not produce radioactive waste, unlike fission.
Mindmap
Keywords
💡Nuclear Fission
💡Nuclear Fusion
💡Chain Reaction
💡Radioactive
💡Isotopes
💡Neutrons
💡Gamma Rays
💡Energy Density
💡Nuclear Reactors
💡Protostar
💡Kindness
Highlights
Nuclear fusion is the process of merging atoms together, whereas nuclear fission involves splitting atoms.
Nuclear fission specifically refers to the splitting of a large atom like uranium-235 into smaller elements.
Uranium-235 becomes unstable when struck by a neutron, leading to the formation of uranium-236 and subsequent fission.
Fission of uranium-236 results in lighter elements, additional neutrons, gamma rays, and energy release.
The energy from nuclear fission is significantly greater than that from chemical reactions, making it a dense energy source.
Nuclear fission can set off a chain reaction, with new neutrons striking other uranium-235 atoms.
Radioactive byproducts such as iodine-131, cesium-137, and strontium-90 are produced during nuclear fission.
Exposure to fission byproducts can cause sickness, cancer, and even death.
Nuclear fission heat can be used to generate electricity through steam heating and turbine rotation.
There are currently 439 nuclear reactors in operation across 30 countries worldwide.
Nuclear fusion involves combining lighter elements, such as deuterium and tritium, to form a heavier one like helium.
Fusion of hydrogen isotopes releases a neutron and a large amount of energy.
Achieving nuclear fusion on Earth requires temperatures of approximately 100 million degrees Celsius.
Nuclear fusion is the energy source of stars and begins when a protostar's core reaches extreme temperatures.
Fusion creates more energy than fission and does not produce radioactive byproducts.
The video concludes with a reminder to practice kindness, emphasizing its multiplying effect.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: