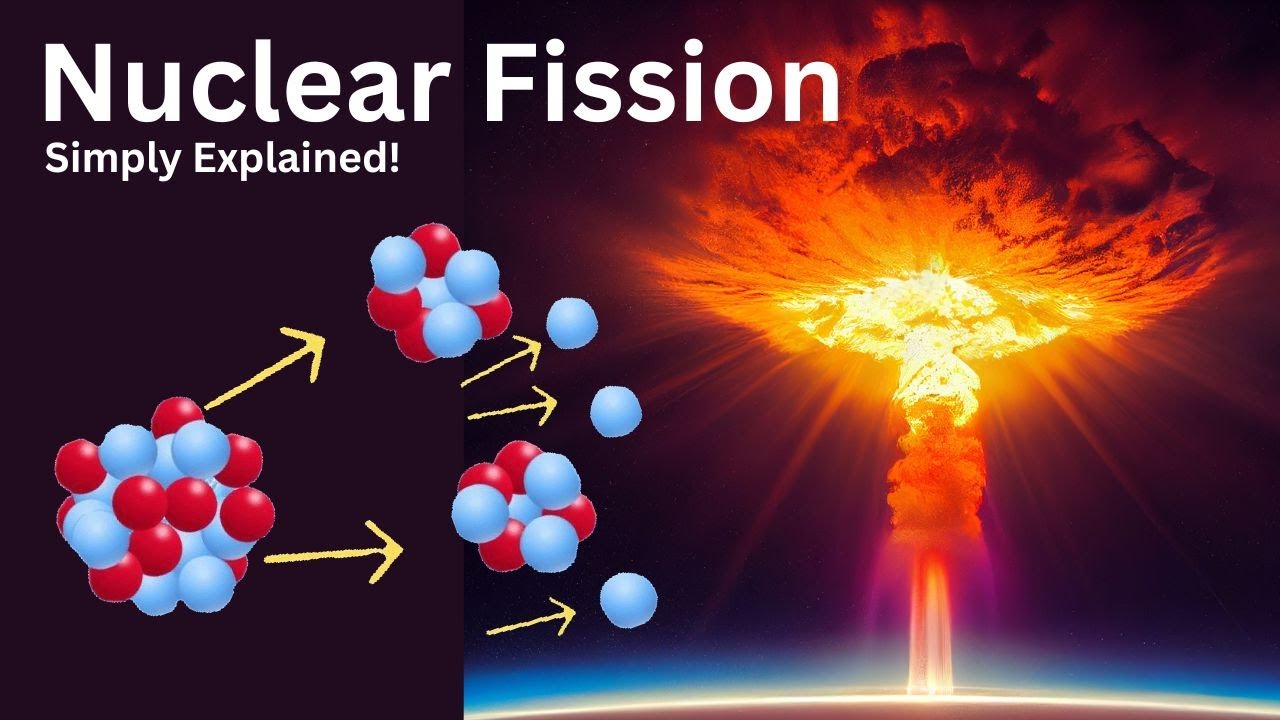
Nuclear fission | High school chemistry | Khan Academy
TLDRThis educational script delves into the principles of nuclear fission, the process that powers both atomic bombs and nuclear power plants. It explains how heavy nuclei like Uranium 235 split into smaller nuclei upon neutron bombardment, releasing energy and additional neutrons. The script clarifies the difference between the uncontrolled chain reactions in bombs and the controlled chain reactions in reactors, which are used to generate electricity without emitting CO2. The enrichment process of uranium is highlighted, with bombs requiring highly enriched uranium for rapid fission, whereas power plants use only 3-5% enriched uranium to maintain a steady energy release. The summary also touches on the challenge of safely disposing of radioactive waste produced in nuclear power plants.
Takeaways
- 🔬 Nuclear fission is the process where a heavy nucleus splits into smaller nuclei, usually after being hit by a neutron.
- 💥 The energy released in nuclear fission comes from the difference in mass between the reactants and products, following Einstein's mass-energy equivalence principle (E=mc²).
- ⚛️ Not all heavy nuclei can undergo fission; only fissile nuclei like Uranium-235 can easily split and release energy.
- 🌐 A chain reaction occurs when the neutrons produced by one fission event cause further fission in other fissile nuclei, leading to an exponential increase in reactions.
- 💣 Atomic bombs utilize uncontrolled chain reactions to release a massive amount of energy rapidly, causing devastating explosions and long-term radioactive contamination.
- ⚡ Nuclear power plants, on the other hand, use controlled chain reactions to generate electricity steadily and safely.
- 🚫 Controlling a chain reaction in a reactor is achieved by absorbing excess neutrons, preventing the reaction from escalating uncontrollably.
- ⚛️ Enrichment is the process of increasing the amount of fissile material in uranium ore; bombs require highly enriched uranium, while reactors use only 3-5% enriched uranium.
- 🌡 In nuclear reactors, the energy from controlled fission reactions is used to heat water, producing steam that drives turbines to generate electricity.
- 🌿 Nuclear power is a more efficient and cleaner energy source compared to fossil fuels, as it does not produce CO2 emissions during energy production.
- 🧼 Radioactive waste is a byproduct of nuclear fission and must be managed and disposed of safely, presenting a significant challenge for nuclear energy.
Q & A
What is nuclear fission?
-Nuclear fission is a nuclear reaction in which a heavy nucleus splits into smaller nuclei. This process often occurs when a neutron is absorbed by an unstable nucleus, causing it to split and release additional neutrons and energy.
How does nuclear fission differ between spontaneous and induced fission?
-Spontaneous fission occurs without any external influence, while induced fission is caused by bombarding a heavy nucleus with a neutron, which pushes it over the edge of stability and causes it to split.
What is the role of neutrons in nuclear fission?
-Neutrons play a crucial role in nuclear fission. They can cause an unstable nucleus to split by being absorbed, and upon splitting, the nucleus releases additional neutrons, which can then induce further fission reactions.
Can all heavy nuclei undergo fission?
-No, not all heavy nuclei can undergo fission. Only certain types, known as fissile nuclei, can readily undergo fission and release energy. Uranium-235 is an example of a fissile nucleus, while Uranium-238 is not.
How can the number of neutrons released in a fission reaction be determined?
-The number of neutrons released in a fission reaction can be determined by balancing the nuclear reaction equation, ensuring the conservation of protons and total nucleons (protons and neutrons).
What is a chain reaction and why is it important in nuclear fission?
-A chain reaction is a sequence of events where each fission reaction releases neutrons that can cause further fission reactions. This process is crucial in nuclear fission as it allows for a self-sustaining series of reactions that can release a significant amount of energy.
Why doesn't a nuclear reactor explode like an atomic bomb?
-A nuclear reactor does not explode like an atomic bomb because it uses a controlled chain reaction. Neutrons are absorbed to regulate the rate of fission reactions, ensuring a steady release of energy rather than a rapid, uncontrolled explosion.
What is the purpose of uranium enrichment in the context of nuclear energy?
-Uranium enrichment is the process of increasing the concentration of fissile isotopes, such as Uranium-235, in the uranium ore. This is necessary because natural uranium contains a small percentage of Uranium-235, which is not sufficient to sustain a chain reaction for power generation or bomb making.
How is the energy produced in a nuclear reactor used to generate electricity?
-The energy produced in a nuclear reactor is primarily in the form of kinetic energy from the fission products and neutrons. This energy heats water to produce high-pressure steam, which drives turbines connected to generators to produce electricity.
What is the difference between the enrichment levels of uranium used in nuclear power plants and atomic bombs?
-Nuclear power plants typically use uranium with an enrichment level of about 3 to 5%, which is not enough to cause an explosion. In contrast, atomic bombs require a much higher enrichment level, often around 90%, to ensure a rapid and powerful chain reaction.
What are the challenges associated with the radioactive waste produced by nuclear power plants?
-The radioactive waste produced by nuclear power plants is hazardous and requires careful handling and disposal. Scientists and engineers are actively working on methods to safely store and manage this waste to prevent environmental contamination and health risks.
Outlines
🔬 Nuclear Fission and its Applications
This paragraph introduces the concept of nuclear fission, which is the process where a heavy nucleus splits into smaller nuclei upon the absorption of a neutron. The lecturer explains that both atomic bombs and nuclear power plants operate based on nuclear fission chain reactions but differ significantly in their application. Uranium 235 is used as an example to illustrate how fission occurs, resulting in the production of smaller elements and additional neutrons. The unpredictability of fission products and the energy released during fission are highlighted, along with the concept that mass is converted into energy in these reactions. The paragraph concludes by differentiating between fissile and non-fissile materials, using Uranium 235 and Uranium 238 as examples, and touches on the immense energy potential of nuclear fission.
🌐 Understanding Chain Reactions in Nuclear Energy
This section delves into how chain reactions are initiated and sustained in nuclear fission. The lecturer describes the process of using neutrons produced by one fission event to trigger further fission events, leading to an exponential increase in reactions and energy release—a chain reaction. The destructive power of atomic bombs, which rely on uncontrolled chain reactions, is contrasted with the controlled chain reactions used in nuclear reactors to generate electricity. The importance of neutron absorption to control the reaction rate is emphasized, as is the process of enriching uranium ore to increase the amount of fissile material. The enrichment levels required for bombs versus nuclear power plants are compared, with the latter using significantly lower enrichment percentages to prevent explosive chain reactions. The paragraph concludes with an explanation of how the kinetic energy from controlled fission reactions is harnessed to produce electricity in a nuclear power plant, similar to conventional power plants but without CO2 emissions.
🌿 The Environmental Impact and Waste Management of Nuclear Power
The final paragraph addresses the environmental impact of nuclear power plants, particularly the management of radioactive waste. The lecturer clarifies a common misconception about cooling towers, explaining that they release only water vapor and do not produce harmful radioactive emissions. However, the need to safely dispose of radioactive waste generated within the nuclear power plant is acknowledged as an ongoing challenge. Scientists and engineers are actively working on solutions to manage this waste, which is a critical aspect of ensuring the long-term sustainability and safety of nuclear energy use.
Mindmap
Keywords
💡Nuclear Fission
💡Chain Reaction
💡Neutron
💡Fissile Nuclei
💡Enrichment
💡Radioactivity
💡Kinetic Energy
💡Mass-Energy Equivalence
💡Controlled Chain Reaction
💡Nuclear Reactor
💡Nuclear Waste
Highlights
Atomic bombs and nuclear power plants operate on the principle of nuclear fission chain reactions.
Nuclear fission is the process where a heavy nucleus breaks into smaller nuclei, often by bombarding it with a neutron.
Uranium 235 can undergo fission and produce different products depending on the neutron bombardment.
The number of neutrons produced in fission can be predicted by balancing protons and total particles in the reaction.
Energy is released during fission as kinetic energy of the products and neutrons, resulting in a mass deficit.
Fissile nuclei like uranium 235 undergo fission and release energy, unlike non-fissile nuclei such as uranium 238.
The energy released from nuclear fission is significantly higher than that from chemical reactions in traditional bombs.
Nuclear fission products are radioactive, leading to long-term contamination after an explosion.
A chain reaction in nuclear fission can be self-sustaining, leading to an exponential increase in reactions.
Atomic bombs utilize uncontrolled chain reactions to release a tremendous amount of energy rapidly.
Nuclear power plants use controlled chain reactions to generate electricity steadily.
Controlling chain reactions involves absorbing neutrons to regulate the rate of fission.
Enrichment is the process of increasing the amount of fissile material in uranium ore.
Bombs require highly enriched uranium, while nuclear reactors use only 3-5% enriched uranium.
In nuclear reactors, the energy from controlled fission reactions is used to heat water and produce steam for electricity generation.
Cooling towers in nuclear power plants release water vapor, not radioactive materials.
Radioactive waste from nuclear power plants must be safely managed and disposed of.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: