Emission and Absorption Spectra
TLDRIn this AP Physics essentials video, Mr. Andersen explores the concepts of emission and absorption spectra. He explains how hydrogen gas, when excited in a discharge chamber, emits discrete units of light corresponding to specific energy levels. This emission spectrum can be analyzed using a prism, revealing the unique colors associated with each energy transition. Conversely, when light is shone through the gas, some wavelengths are absorbed, creating an absorption spectrum. Bohr's model of the atom helps explain these phenomena, where electrons absorb or emit photons as they transition between energy levels. The video also discusses the broader spectrum, including infrared and ultraviolet light, and illustrates how different elements produce distinct spectral lines, which are crucial for identifying the composition of substances.
Takeaways
- 🌟 Emission and absorption spectra are key concepts in understanding atomic behavior. When a gas like hydrogen is excited by electrons in a discharge chamber, it emits light with discrete wavelengths, creating an emission spectrum.
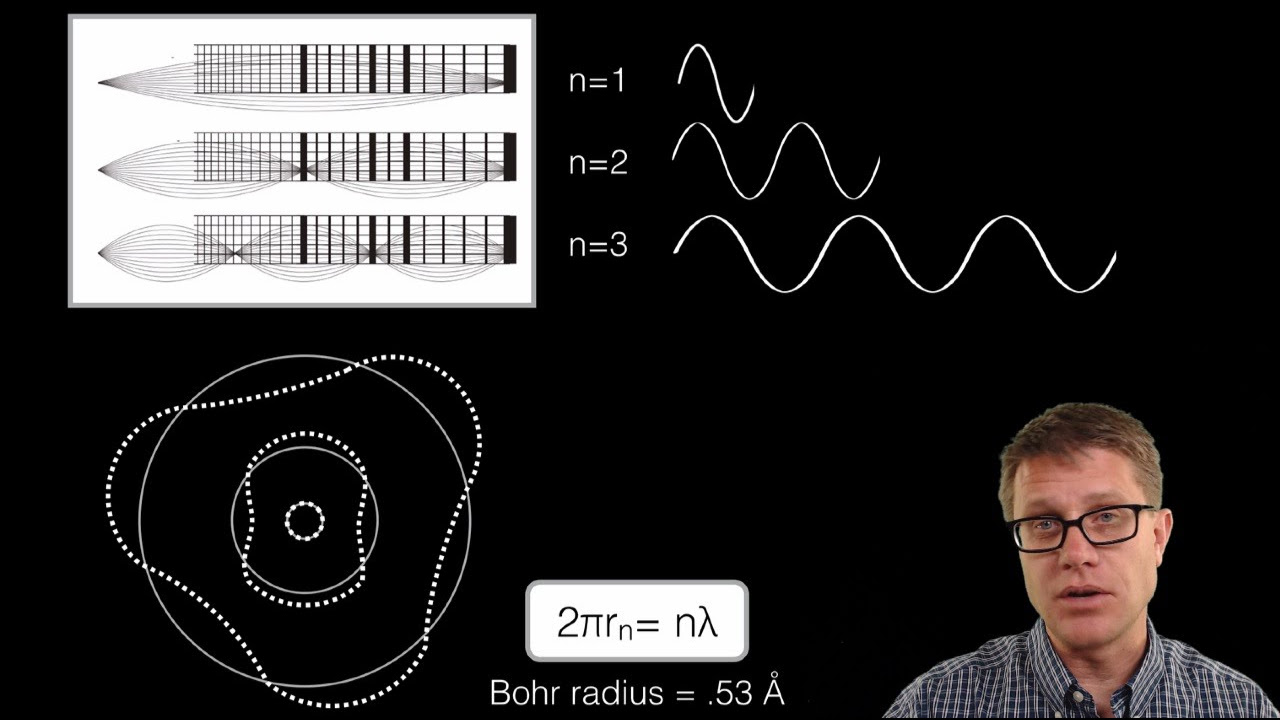
- 🔬 The discrete units of light observed in the emission spectrum are due to the quantized energy levels of the hydrogen atom. Electrons can only transition between these levels, emitting or absorbing photons with specific energies.
- 🌈 When analyzing light through a prism, the light is split into its constituent colors, demonstrating the spectrum of visible light. However, there are also invisible parts of the spectrum, such as infrared and ultraviolet light.
- 💡 Niels Bohr's model of the atom explains the emission and absorption spectra. Electrons in a hydrogen atom jump between energy levels, emitting or absorbing photons with energy corresponding to the difference between those levels.
- 🚫 Only photons with the correct energy can cause an electron to transition to a higher energy level. If the photon's energy does not match the energy difference between levels, it will not be absorbed.
- 🔄 Conservation of energy is fundamental in both absorption and emission processes. The energy of the photon and the atom before absorption equals the energy inside the atom after absorption, and similarly for emission.
- 🔍 Scientists can identify elements and molecules by analyzing their emission spectra. Each element has a unique set of energy levels, resulting in a distinctive pattern of spectral lines.
- 🌌 The visible light spectrum is just a small part of the full electromagnetic spectrum. Beyond visible light, there are infrared and ultraviolet regions, each with its own applications and properties.
- 💥 In the video's simulation, a continuous stream of electrons hitting hydrogen atoms results in the emission of photons of various colors, demonstrating how different energy transitions produce different wavelengths of light.
- 🌈 The simulation also shows how different atoms, such as mercury or neon, have different energy levels and thus produce different spectral lines, leading to their characteristic colors when used in lights.
- 📊 A spectrogram can visually represent the spectral lines, showing the specific wavelengths emitted or absorbed by an atom, which is crucial for identifying the composition of celestial bodies or chemical substances.
Q & A
What happens when a gas like hydrogen is placed in a discharge chamber and electrons are shot at it?
-The gas will give off light that can be analyzed and split into discrete units of light, or photons, carrying discrete amounts of energy when passed through a prism.
What is the significance of the discrete units of light observed in the emission spectrum of hydrogen?
-The discrete units of light indicate specific energy levels that electrons can occupy in a hydrogen atom, and they are the result of electrons transitioning between these levels.
What is an absorption spectrum and how does it differ from an emission spectrum?
-An absorption spectrum is the result of shining light through a medium where certain parts of the light are absorbed, unlike an emission spectrum where light is emitted by atoms or molecules as they transition between energy levels.
Who is credited with the explanation of the emission and absorption spectra of hydrogen?
-Niels Bohr is credited with providing the explanation for the emission and absorption spectra of hydrogen, relating them to electron transitions between energy levels.
How does the energy conservation principle apply to the absorption and emission of light by atoms?
-According to the energy conservation principle, the amount of energy in the photon and the atom or nucleus before absorption is equal to the amount of energy inside the atom or nucleus after absorption. Similarly, the energy in the atom or nucleus before emission is equal to the energy of the emitted photon plus the energy of the atom or nucleus after emission.
Why is the study of emission and absorption spectra valuable in science?
-The study of emission and absorption spectra is valuable because it allows scientists to identify the elements and molecules present in a sample by analyzing the specific wavelengths of light that are emitted or absorbed, which correspond to the energy levels of the electrons in those substances.
What is the relationship between visible light and the spectrum of light that is produced when it passes through a prism?
-Visible light encompasses all the colors of the spectrum, which are produced when white light passes through a prism and is split into its constituent wavelengths. This visible spectrum is flanked by infrared and ultraviolet light, which are not visible to the human eye.
What happens to an electron in a hydrogen atom when it absorbs a photon of the correct color?
-When an electron in a hydrogen atom absorbs a photon of the correct color, it gains energy and jumps to a higher energy level.
How does the color of light emitted by an electron relate to the energy level transition it undergoes?
-The color of light emitted by an electron is directly related to the energy level transition it undergoes. When an electron falls from a higher energy level to a lower one, it emits a photon of light with a color corresponding to the energy difference between those levels.
What is a PHET simulation and how is it used in the context of the script?
-A PHET simulation is an interactive computer model used for education, often to visualize and explore scientific concepts. In the context of the script, a PHET simulation is used to demonstrate the emission of light from a hydrogen atom when it is excited by a stream of electrons.
How do different gases, like mercury or neon, produce different colors in a gas discharge tube?
-Different gases have different electron energy levels, which result in different colors of light being emitted when the electrons transition between these levels. For example, neon gas emits a reddish color due to the specific energy levels of its electrons and the corresponding photons emitted during transitions.
Outlines
🌟 Emission and Absorption Spectra in Physics
Mr. Andersen introduces the concept of emission and absorption spectra in the context of atomic physics. He explains that when hydrogen gas is placed in a discharge chamber and bombarded with electrons, it emits light that, when passed through a prism, reveals discrete units of light corresponding to specific energy levels. This phenomenon was historically puzzling until Niels Bohr's quantum theory provided an explanation. The video also discusses how shining light through a discharge chamber results in an absorption spectrum, where certain colors of light are absorbed by the gas. The conservation of energy is highlighted as a fundamental principle in these processes, with the energy of the photon being equal to the energy change within the atom during absorption or emission. This understanding is crucial for analyzing the composition of elements and molecules based on their unique spectral signatures.
Mindmap
Keywords
💡Emission and Absorption Spectra
💡Discrete Units of Light
💡Niels Bohr
💡Conservation of Energy
💡Energy Levels
💡Spectrogram
💡Infrared and Ultraviolet Light
💡PHET Simulation
💡Electron Transitions
💡Sodium and Neon
💡Quantum Mechanics
Highlights
Emission and absorption spectra are discussed in the video.
Hydrogen gas in a discharge chamber emits light when electrons are shot at it.
Light emitted from hydrogen can be analyzed through a prism to show discrete units of light.
Absorption spectra occur when light is shone through a discharge chamber and some is absorbed.
Niels Bohr's theory explains the emission and absorption of light by hydrogen atoms.
Electrons emit photons when they jump to lower energy levels.
Electrons absorb photons to move to higher energy levels.
Only discrete colors of light are required for electron transitions.
Conservation of energy is observed in both absorption and emission of light.
Emission spectra can reveal the elements present in a substance by analyzing the light given off.
Visible light is part of the spectrum, with infrared and ultraviolet light outside the visible range.
Energy level diagrams for hydrogen atoms show how specific colors of photons affect electron transitions.
Different atoms, such as mercury and neon, have unique emission spectra.
Sodium and neon gases emit specific colors of light due to their atomic structure.
Neon lights produce a reddish color from the sum of photons emitted by neon gas.
Understanding emission and absorption spectra helps in identifying elements and their energy transitions.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: