Introduction to Moles
TLDRThe video script provides an educational overview of the concept of moles in chemistry. It clarifies that a mole is a unit representing 602 hexillion (written as 6.02 x 10^23) of a specific entity, similar to a dozen representing 12 items. The video emphasizes that moles are not a shorthand for molecules, but rather a way to quantify vast numbers of entities, which can be atoms, molecules, or even larger objects like jellybeans or doughnuts for illustrative purposes. The script also explains the use of scientific notation to simplify the representation of large numbers like Avogadro's number, which is the number of entities in a mole. Finally, it illustrates the concept by comparing the immense size of a mole of jellybeans to the more manageable size of a mole of sulfur atoms, highlighting the minuscule scale of atoms.
Takeaways
- 🧪 A mole is a unit in chemistry that represents a specific number of things, much like a dozen represents 12 items.
- 🔢 The number of things in a mole is 6.02 x 10^23, which is also known as Avogadro's number, named after the Italian scientist who discovered it.
- 🐭 Despite the name, a mole is not an abbreviation for 'molecule'; it is a group of 6.02 x 10^23 entities, which could be atoms, molecules, or any other particles.
- 📏 A mole can be used to measure any type of particles, not just atoms or molecules, as long as the count is 6.02 x 10^23 of them.
- 📉 The concept of a mole is useful because it simplifies the representation of very large numbers, making calculations and notations more manageable.
- ✍️ The number 6.02 x 10^23 is often written in scientific notation as 6.02, which is much easier to work with in mathematical expressions.
- 🌍 To illustrate the magnitude of Avogadro's number, if you had 602 hexillion jellybeans, they would be as large as the entire planet Earth.
- 🚀 If you had 602 hexillion doughnuts and stacked them, the stack would reach from the Earth to the Sun and back 200 billion times.
- 🔬 In contrast to the macroscopic examples, a mole of atoms, such as sulfur atoms, is much smaller and can fit in a relatively small container, demonstrating the tiny size of atoms.
- 🧐 The use of moles in chemistry allows scientists to compare and measure the amounts of different substances on a molecular or atomic level, despite their vastly different physical sizes.
- ⚖️ Understanding moles is fundamental to performing stoichiometry in chemistry, which is essential for chemical analysis and reactions.
Q & A
What is a mole in the context of chemistry?
-A mole in chemistry is a way to express a specific number of entities, like atoms or molecules. It is not a reference to the small furry creature. It is similar to a dozen but much larger, representing 6.02 times 10 to the 23rd entities.
How many entities are there in a mole?
-There are 6.02 times 10 to the 23rd entities in a mole, which is often referred to as Avogadro's number.
What is the significance of Avogadro's number?
-Avogadro's number is significant because it represents the number of entities in one mole. It is named in honor of the Italian scientist Amedeo Avogadro who discovered it.
How is the number 602 hexillion abbreviated in scientific notation?
-The number 602 hexillion is abbreviated in scientific notation as 6.02 times 10 to the 23rd.
Why is scientific notation used to express large numbers like Avogadro's number?
-Scientific notation is used to express large numbers like Avogadro's number because it simplifies the number, making it easier to handle in mathematical calculations and avoiding the need to write out all the zeros.
What is the difference between a mole and a molecule?
-A mole is a unit representing a specific number of entities, which could be atoms, molecules, or any other particles, while a molecule is a group of two or more atoms bonded together.
Can you have a mole of any substance?
-Yes, the concept of a mole can apply to any substance. It is a way to quantify a very large number of entities, whether they are doughnuts, jellybeans, or atoms.
How does the size of a mole of atoms compare to a mole of larger objects like jellybeans?
-A mole of larger objects like jellybeans would be as large as the entire planet Earth, whereas a mole of atoms is much smaller due to the tiny size of atoms. For example, a mole of sulfur atoms can fit in a small dish.
Why do people sometimes confuse a mole with a molecule?
-People sometimes confuse a mole with a molecule because the term 'mole' sounds similar to 'molecule' and they might not be aware that a mole is a unit representing a vast number of entities, not a single entity.
What is an example of how large a mole of jellybeans would be?
-A mole of jellybeans, which is 6.02 times 10 to the 23rd jellybeans, would be as large as the entire planet Earth.
How does the size of a mole of atoms differ from a mole of macroscopic objects?
-A mole of atoms is much smaller compared to a mole of macroscopic objects due to the minuscule size of atoms. For instance, a mole of sulfur atoms can fit in a small dish, whereas a mole of jellybeans or doughnuts would be astronomically larger.
What is the practical application of understanding moles in chemistry?
-Understanding moles in chemistry is crucial for performing calculations related to chemical reactions, determining the amount of reactants and products, and for understanding the stoichiometry of chemical equations.
Outlines
🌟 Understanding Moles in Chemistry
The video introduces the concept of moles, which are a fundamental unit in chemistry representing a specific, incredibly large number of entities—602 hexillion, also known as Avogadro's number. It clarifies that a mole is not an abbreviation for 'molecule' but rather a count of 602 hexillion of anything, whether it be atoms, doughnuts, or jellybeans. The video also explains the use of scientific notation to represent this large number as 6.02 times 10 to the 23rd, making it more manageable for mathematical operations.
🌍 The Immensity of a Mole
This paragraph delves into the magnitude of a mole by using the example of jellybeans. It illustrates that 602 hexillion jellybeans would equate to the size of the entire planet Earth, emphasizing the vastness of a mole. The video further explains that while a mole of larger items like doughnuts would stretch from Earth to the Sun and back 200 billion times, a mole of atoms, despite being the same quantity, is much smaller due to the minuscule size of atoms. It concludes by noting that while the number 602 hexillion is immense, the actual space occupied by a mole of atoms is quite manageable.
🔢 Avogadro's Number and Molar Representation
The final paragraph reinforces the concept of Avogadro's number, which is the number of entities in a mole—602 hexillion. It reiterates the different ways this number can be expressed, including the full numerical form with 21 zeros, the term 'hexillion,' and the scientific notation of 6.02 times 10 to the 23rd. The video concludes by highlighting the practicality of using scientific notation for such a large number and by reminding viewers of the key points discussed about moles, their size, and their application in chemistry.
Mindmap
Keywords
💡Mole (chemistry)
💡Avogadro's number
💡Scientific notation
💡Stoichiometry
💡Mole (unit)
💡Chemical reaction
💡Atoms
💡Scale
💡Jellybeans
💡Doughnuts
💡Sulfur atoms
Highlights
A mole is a specific number of things, similar to a dozen but much larger, containing 6.02 x 10^23 entities.
The term 'mole' should not be confused with 'molecule'; a mole is a collection of 6.02 x 10^23 molecules.
The number 6.02 x 10^23 is known as Avogadro's number, named after the Italian scientist who discovered it.
Moles can represent any substance, not just atoms, and can be visualized with examples like doughnuts or jellybeans for easier understanding.
The concept of a mole is fundamental in chemistry, allowing for a standardized way to count and measure large quantities of particles.
Avogadro's number can be daunting due to its size, but it simplifies calculations when dealing with very large numbers of particles.
Scientific notation is used to abbreviate large numbers like Avogadro's number, making it more manageable for mathematical operations.
The mole is a bridge between macroscopic and microscopic scales in chemistry, providing a way to quantify substances in a laboratory.
The size of a mole of atoms is much smaller than a mole of larger objects like jellybeans, demonstrating the tiny scale of atoms.
A mole of sulfur atoms can fit in a small dish, contrasting sharply with the immense size a mole of jellybeans would occupy.
The concept of moles helps to understand the vast differences in scale between different types of substances.
A mole of any substance, from jellybeans to atoms, contains the same number of entities: 6.02 x 10^23.
Visualizing a mole as a stack of doughnuts reaching from Earth to the Sun underscores the immense scale of a mole.
Despite the large numerical value, the physical space occupied by a mole of atoms is relatively small due to their minuscule size.
Understanding moles is crucial for chemical calculations and stoichiometry, providing a consistent measure for chemical reactions.
The mole is a fundamental unit in the International System of Units (SI), essential for scientific communication and standardization.
The video provides an accessible introduction to the concept of moles, making complex chemical principles more understandable.
Transcripts
Browse More Related Video

The MOLE & Avogadro's Number (Chemistry)

What is a Mole? | #Extraclass #MoleConcept #Chemistry #Animation

Introduction to Moles

The mole and Avogadro's number | Atoms, compounds, and ions | Chemistry | Khan Academy
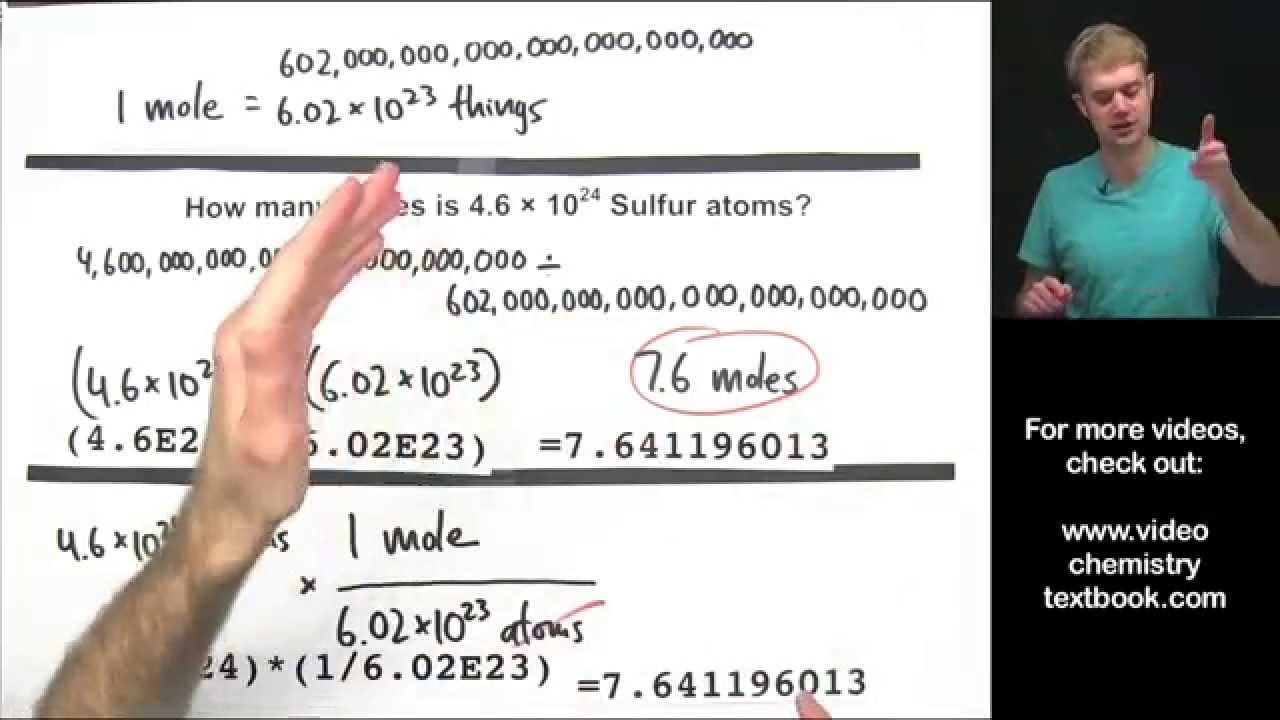
Converting Between Moles, Atoms, and Molecules

Avogadro's Number, The Mole, Grams, Atoms, Molar Mass Calculations - Introduction
5.0 / 5 (0 votes)
Thanks for rating: