Converting Between Moles, Atoms, and Molecules
TLDRThis educational video script teaches viewers how to convert between moles and the number of atoms or molecules. It simplifies the concept by comparing moles to more familiar units like dozens and explains the Avogadro's number, which is 6.02 x 10^23 atoms per mole. The script walks through sample problems, demonstrating both a straightforward method and the use of conversion factors. It also emphasizes the importance of scientific notation and significant figures in calculations. The video aims to clarify common confusions about moles and conversion factors, providing clear steps for converting from moles to atoms and vice versa.
Takeaways
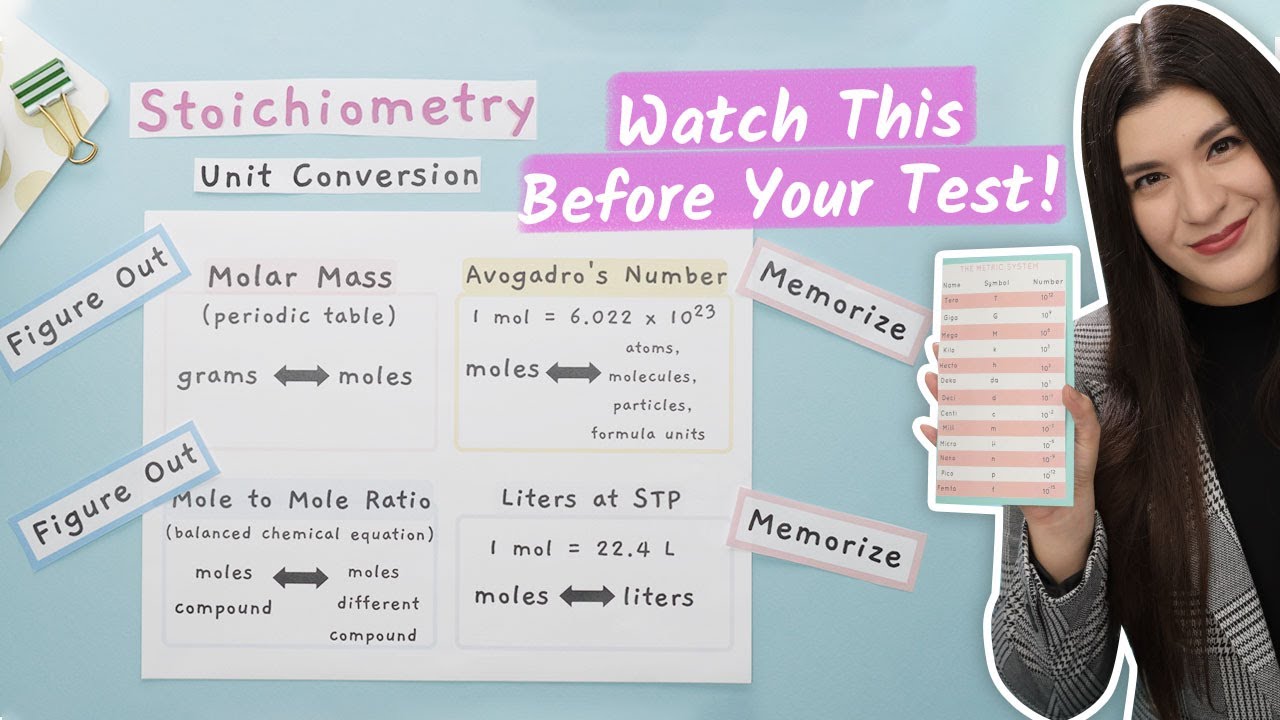
- 🔬 Conversion between moles and atoms/molecules involves understanding the concept of a mole, which is a unit amount of a chemical substance, containing 6.02 x 10^23 representative particles.
- 🧐 Particles can refer to any individual entities such as atoms, molecules, or even non-scientific items like jellybeans or coins.
- 📚 The video demonstrates two methods for converting between moles and atoms: a straightforward method and the use of conversion factors.
- 🤔 The straightforward method involves simple multiplication by Avogadro's number (6.02 x 10^23) to convert moles to atoms or molecules.
- 📉 Conversion factors are a common tool in chemistry for unit conversions, and the video explains how to use them to convert between moles and atoms.
- 🔢 When using conversion factors, it's crucial to set up the factor correctly to cancel out the unit you want to eliminate (e.g., moles) and solve for the desired unit (e.g., atoms).
- 💡 Scientific notation is used to simplify calculations involving large numbers, such as those in mole calculations, and is typically entered into calculators using the 'E' notation for exponents.
- ✂️ Significant figures are important in scientific calculations to ensure the precision of the result, and the video shows how to round numbers to the correct number of significant figures.
- 📉 The video provides a step-by-step guide on how to perform calculations using both methods, starting with an example of converting 5.5 moles to atoms.
- 🔄 The process is also demonstrated in reverse, showing how to convert a large number of atoms (4.6 x 10^24 sulfur atoms) back into moles.
- 📚 The script emphasizes the importance of understanding the concepts behind mole calculations and provides an approachable way to tackle these problems, even for those who find conversion factors confusing.
Q & A
What is the basic concept of a mole in chemistry?
-A mole is a unit used in chemistry to express amounts of a chemical substance, similar to a dozen in everyday language. It represents 6.02 x 10^23 individual entities, which can be atoms, molecules, ions, or other particles.
How are moles and particles related in the context of the video?
-In the video, particles refer to any individual entities such as atoms or molecules. The concept of a mole is used to count the number of these particles in a given sample, with one mole being equivalent to 6.02 x 10^23 particles.
What is the purpose of using conversion factors in chemistry?
-Conversion factors are used in chemistry to convert between different units of measurement, such as from moles to atoms or vice versa. They are particularly useful for solving stoichiometry problems and are often emphasized in educational materials despite some students finding them confusing.
How does the video explain the conversion from moles to atoms?
-The video explains the conversion from moles to atoms by comparing it to a more familiar concept, like dozens. It then demonstrates the process using scientific notation and a scientific calculator, emphasizing the use of the Avogadro's number (6.02 x 10^23) to find the number of atoms in a given number of moles.
What is the significance of Avogadro's number in the mole concept?
-Avogadro's number, which is approximately 6.02 x 10^23, is the number of particles (atoms, molecules, ions, etc.) in one mole of a substance. It is used as a conversion factor to relate the macroscopic amount of a substance (in moles) to the number of particles it contains.
How does the video demonstrate the use of scientific notation in mole calculations?
-The video demonstrates the use of scientific notation by converting the large number 602 hexillion into 6.02 x 10^23, which is easier to work with, especially on a scientific calculator. It shows how to input this into a calculator and emphasizes the importance of using scientific notation for complex numbers.
What is the process of converting atoms to moles as shown in the video?
-The process involves dividing the number of atoms by Avogadro's number (6.02 x 10^23). The video shows this as a division problem, which can be solved using a calculator, and then rounding the result to the appropriate number of significant figures.
How does the video handle significant figures in mole calculations?
-The video explains that when performing mole calculations, one should round the final answer to the number of significant figures present in the original numbers used in the calculation. It demonstrates this by rounding the calculated number of atoms to moles to two significant figures.
What alternative method does the video suggest for those who cannot use a calculator?
-The video mentions that for those who cannot use a calculator, there is an alternative method demonstrated in another video on doing mole calculations by hand.
How does the video use conversion factors to convert moles to atoms and vice versa?
-The video shows how to create conversion factors from the definition of a mole and Avogadro's number. It uses these factors to cancel out the unit of moles, leaving the unit of atoms or vice versa, and then performs the calculation by multiplying or dividing as necessary.
What is the importance of understanding the concept of moles in relation to atoms and molecules?
-Understanding the concept of moles in relation to atoms and molecules is crucial for stoichiometry, which is the calculation of quantities in chemical reactions. It allows chemists to predict and measure the amounts of reactants and products in a reaction based on the mole concept.
Outlines
🔍 Introduction to Mole-Atom Conversion
The video begins with an introduction to converting between moles and the number of atoms or molecules. The concept of a 'particle' is explained as any individual entity, such as an atom or molecule. The video promises to demonstrate two methods for these conversions: a straightforward approach and the use of conversion factors. The mole is likened to a dozen, with 6.02 x 10^23 particles in a mole, which is the Avogadro's number. The first problem involves converting 5.5 moles of atoms to the number of atoms, using a simple analogy with dozens and then applying the concept to moles. The Avogadro's number is used to perform the calculation, and the importance of scientific notation and significant figures is highlighted.
📚 Calculating Atoms from Moles Using Conversion Factors
This paragraph delves into solving the same problem of converting moles to atoms, but this time using conversion factors. The concept of a conversion factor is introduced, which is a ratio that allows for unit cancellation and conversion between units. The Avogadro's number is used to create two possible conversion factors. The correct conversion factor is chosen to cancel out the mole unit and solve for the number of atoms. The process involves multiplying 5.5 moles by Avogadro's number and dividing by one mole to ensure the mole unit cancels out. The calculation is performed, and the result is presented in scientific notation, with an emphasis on rounding to the correct number of significant figures.
🔢 Converting Atoms to Moles: Calculations and Conversion Factors
The final paragraph addresses the reverse problem: converting a large number of atoms to moles. The analogy of dozens is used again to simplify the concept, but the focus shifts to using moles as the unit. The number of atoms in one mole (Avogadro's number) is used to divide the total number of atoms to find the number of moles. The calculation is shown in scientific notation, and the importance of significant figures in rounding the final answer is reiterated. Additionally, the paragraph explains how to use conversion factors for the same conversion, emphasizing the cancellation of units to solve for moles. The video concludes by directing viewers to additional resources for more practice on these types of problems.
Mindmap
Keywords
💡Moles
💡Atoms
💡Particles
💡Conversion Factors
💡Scientific Notation
💡Significant Figures
💡Calculator
💡Avogadro's Number
💡Conversion Factor Equation
💡Mole Calculations
Highlights
Introduction to converting between moles and number of atoms or molecules.
Explanation of the term 'particle' as a general term for any individual thing, including atoms and molecules.
Approach to converting moles to atoms using a simple, straightforward method.
Introduction of conversion factors as a method for calculations, despite potential confusion for students.
Illustration of the concept of a mole with an analogy to a dozen, highlighting 602 hexillion atoms in a mole.
Demonstration of converting 5.5 moles of atoms to the number of atoms using multiplication.
Use of scientific notation (6.02 x 10^23) for easier calculation of moles to atoms conversion.
Explanation of how to input numbers in scientific notation into a calculator.
Conversion of the calculator result into standard scientific notation for clarity.
Emphasis on the importance of significant figures in rounding the final answer.
Final result of 3.3 x 10^24 atoms from 5.5 moles of atoms.
Introduction of an alternative method using conversion factors for mole to atom conversion.
Step-by-step guide on writing and using conversion factors for mole to atom conversion.
Reinforcement of the concept that conversion factors are derived from the definition of a mole.
Conversion of a large number of sulfur atoms to moles using a common sense approach.
Explanation of dividing a large number of atoms by Avogadro's number (6.02 x 10^23) to find moles.
Demonstration of using scientific notation for the conversion of atoms to moles.
Final result of 7.6 moles of sulfur atoms from a given number of atoms.
Introduction of conversion factors for the reverse process, from atoms to moles.
Guide on selecting the appropriate conversion factor for atoms to moles conversion.
Final demonstration of the calculation process using both direct division and conversion factors.
Encouragement to watch the next video for further practice on mole, atom, and molecule conversions.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: