Lesson 5 - Density in Chemistry
TLDRIn this engaging video, chemistry tutor Jason introduces the concept of density, a fundamental physical property that is crucial for understanding various elements and compounds. He explains that density is commonly associated with the idea of something being heavy or hard to penetrate, but in scientific terms, it is more accurately defined as mass per unit volume. Jason emphasizes the importance of considering both mass and volume when comparing substances, as mass alone is not a sufficient descriptor of a material's properties. He illustrates this by contrasting the mass of a small pile of lead with that of a larger quantity, highlighting how volume plays a critical role in defining density. The video aims to demystify the term 'density', ensuring viewers grasp its significance and application in chemistry.
Takeaways
- 📚 Density is a fundamental concept in chemistry that describes a physical property of matter.
- 🌐 The term 'density' is also used in everyday language, often referring to how compact or packed something is.
- 🧠 In scientific terms, density is related to the mass of an object, but it also involves the concept of volume.
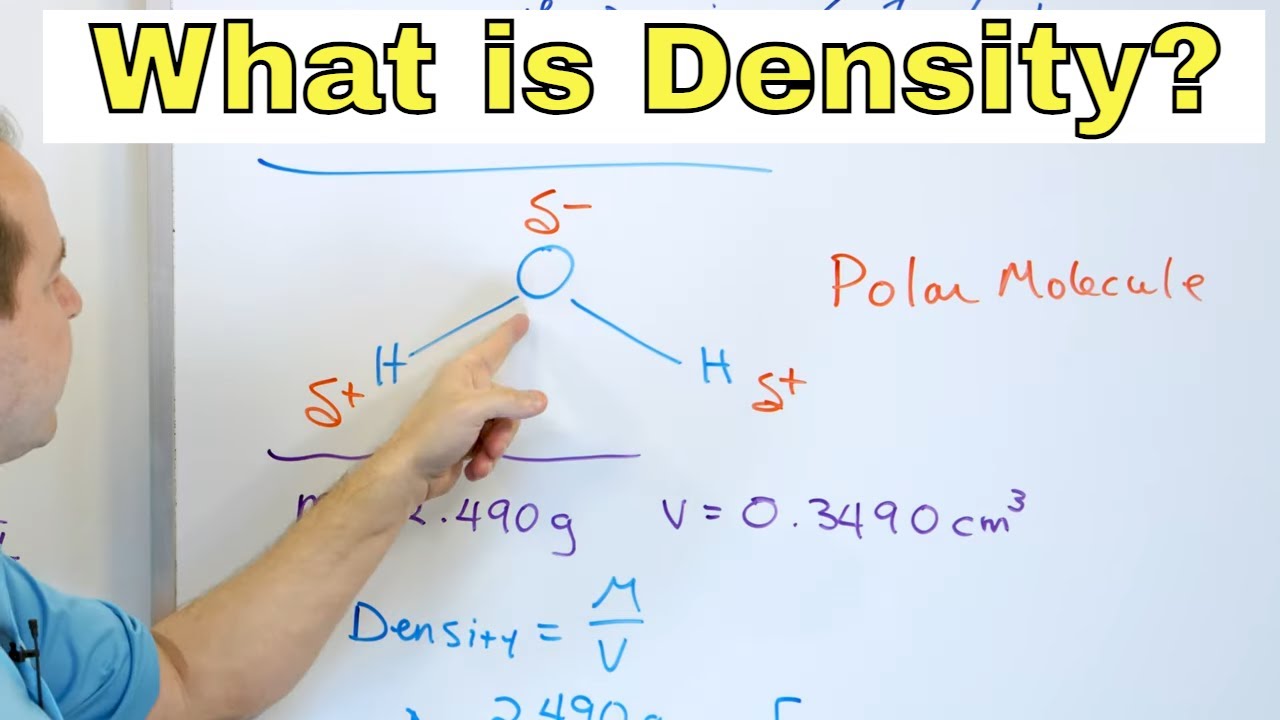
- ⚖️ Density is calculated by dividing the mass of an object by its volume, resulting in mass per unit volume.
- 📏 Understanding density helps to compare how much mass an object has relative to its size.
- 🔍 Density is not just about the mass of an object, but also about the space it occupies.
- 📏 An object's mass alone is not a complete property; it must be considered in relation to its volume.
- 🧮 The density formula is mass divided by volume, which can be represented as Density = Mass/Volume.
- 📦 The mass of an object is useful to know, but it's not the whole story without considering the volume.
- 🏗️ Comparing two objects requires considering both mass and volume to understand their densities.
- 🔑 Density is expressed in units like kilograms per cubic meter (kg/m³) or grams per cubic centimeter (g/cm³).
Q & A
What is density in the context of chemistry?
-Density in chemistry is a physical property that indicates how much mass a substance has relative to its volume. It is expressed as mass per unit volume, typically in grams per cubic centimeter or kilograms per cubic meter.
Why is density considered an important property in chemistry?
-Density is important because it helps in identifying substances and understanding their composition and behavior. It's a key factor in calculations and applications involving mass and volume, such as mixing substances, determining buoyancy, and designing chemical processes.
How can density be measured?
-Density can be measured by dividing the mass of an object by its volume. The mass is usually measured using a balance, and the volume can be determined by calculation or displacement methods depending on the shape and state of the material.
What everyday analogy is used in the script to explain the concept of density?
-The script uses everyday language comparing density to understanding something that is 'dense' like a book or a conversation, implying that something dense has a lot of 'content' or 'substance' packed into a small space, similar to a lot of mass in a small volume.
How does the script differentiate between mass and density?
-The script clarifies that while mass alone can indicate how heavy an object is, it doesn't provide a complete picture without considering volume. Density, therefore, offers a more informative measure by combining both mass and volume to describe how tightly mass is packed in a space.
What examples are given in the script to illustrate the concept of density?
-The script mentions lead and feathers as examples. Lead is described as very dense due to its significant mass in a small volume, whereas feathers are described as less dense because they have little mass even though they might occupy a larger volume.
What practical application of density is suggested in the script?
-A practical application of density mentioned in the script is comparing materials like lead and feathers not just by their mass, but by how much space they take up relative to their mass. This is crucial in fields like material science, engineering, and environmental studies.
Why does the script emphasize the importance of understanding density for students?
-The script emphasizes the importance as density will frequently appear in studies concerning different elements and compounds. Understanding density helps students not to be daunted by it and grasp further chemical concepts more comfortably.
How does the instructor in the script plan to make students understand density?
-The instructor plans to use simple terms and everyday examples to make the concept of density relatable and easier to understand, reinforcing this with mathematical calculations of mass and volume.
What is the role of mass and volume in determining density according to the script?
-According to the script, mass and volume are critical components in determining density. Mass is how much matter an object contains, while volume is the amount of space it occupies. Density is the ratio of these two properties.
Outlines
🌟 Introduction to Density
In this introductory paragraph, Jason, the chemistry tutor, welcomes viewers to a lesson on density. He emphasizes the importance of density as a fundamental physical property that is frequently encountered when studying elements and compounds. Jason explains that while density is a term used in everyday language, it has a specific scientific definition. He prompts viewers to consider their understanding of density and suggests that it often relates to the concept of mass or weight. The paragraph sets the stage for a deeper exploration of density, aiming to ensure that viewers will not be confused or surprised by the term when they encounter it in their studies.
Mindmap
Keywords
💡Density
💡Physical Property
💡Mass
💡Volume
💡Mass per Volume
💡Elements and Compounds
💡Lead
💡Feathers
💡Balance
💡Cylinder
💡Calculation
Highlights
Density is a physical property of everything around us and is important for understanding elements and compounds.
In everyday language, 'dense' usually refers to something being hard to understand or heavy.
Science defines 'dense' as having mass, such as lead being dense due to its weight.
Feathers are considered not dense because they are lightweight.
Density is mass per volume, which is calculated by dividing mass by volume.
Density indicates how much mass something has compared to its size.
Knowing the mass of an object is useful but not sufficient without considering its volume.
The property of a substance is better understood by considering mass in relation to the space it occupies.
Density is the term used to describe mass per volume, providing a more comprehensive understanding of a substance's property.
Objects have mass, which can be measured in grams or kilograms, and also have a volume.
The volume of an object can be calculated if its shape and dimensions are known.
Understanding density is crucial for comparing different substances or materials.
Mass alone does not give a complete picture of a substance's property; volume must also be considered.
Density is a fundamental concept in chemistry that helps in distinguishing between various elements and compounds.
The concept of density is applicable in everyday life and scientific studies.
The tutorial aims to demystify the concept of density, making it accessible and understandable.
By understanding density, one can better appreciate the properties and behaviors of different materials.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: