Lesson 11 - Overview Of The Periodic Table of Elements
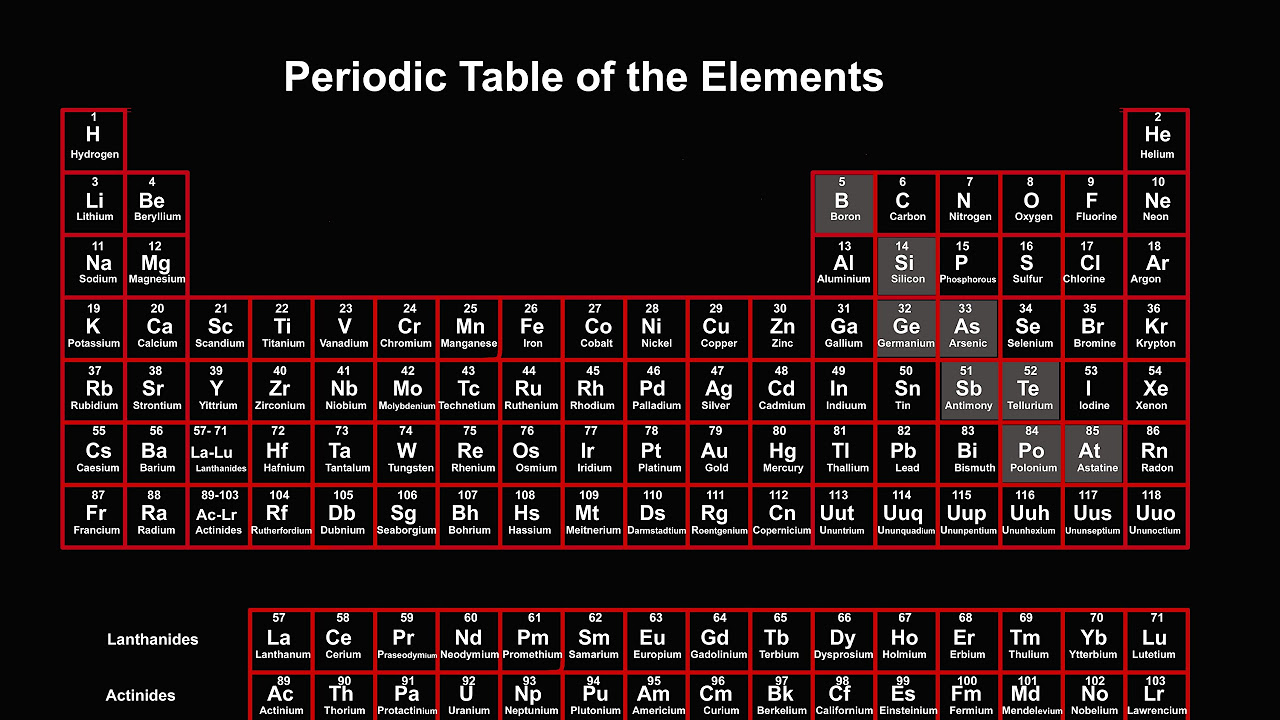
TLDRIn this chemistry tutorial segment, Jason introduces the audience to the periodic table, emphasizing its importance in understanding the organization of elements. He explains that each element has a bold chemical symbol, such as H for hydrogen or He for helium, which is crucial for discussing chemical compounds. Above each symbol is the atomic number, indicating the number of protons in the nucleus and uniquely identifying the element. The atomic mass, often found below the symbol, represents the mass of an element in atomic mass units. Jason encourages students to familiarize themselves with their periodic table, as it will be an essential tool throughout their chemistry studies. The video promises a deeper dive into the periodic table's intricacies later in the course, but for now, focuses on these fundamental aspects.
Takeaways
- 📚 The periodic table is a fundamental tool in chemistry, which organizes elements in a specific way.
- 🔍 Each element on the periodic table has a unique chemical symbol, usually in bold and centered.
- ⚛️ The atomic number, found above the chemical symbol, represents the number of protons in the nucleus of an atom.
- 🧬 The atomic number is key to identifying elements, as each element has a unique number of protons.
- 💧 For example, hydrogen (H) has an atomic number of 1, helium (He) has 2, and so on, indicating the number of protons.
- 📏 The atomic mass, often a decimal and found below the chemical symbol, represents the mass of an element in atomic mass units.
- 🔢 Older chemistry books may refer to atomic mass as atomic weight, but they refer to the same concept.
- 🌐 The layout of the periodic table allows for quick identification of elements based on their atomic number and mass.
- 🔬 Memorizing the symbols and understanding their relation to atomic numbers and masses is crucial for working with chemical compounds.
- 💧 The symbol H2O is used to represent water, demonstrating how chemical symbols are used to denote compounds.
- 📈 As the course progresses, more detailed exploration of the periodic table and its implications will be covered.
- 📘 It's important for students to have their own periodic table for reference during tests, as that's what they will likely use.
Q & A
What is the purpose of this section in the chemistry tutorial?
-This section aims to provide a comprehensive understanding of the periodic table, focusing on its organization and layout, and how to effectively use it as a tool in studying chemistry.
What are the basic elements listed on the periodic table that the script mentions?
-The script mentions hydrogen (H), oxygen (O), helium (He), carbon (C), and their placement and properties on the periodic table.
What is the significance of the atomic number on the periodic table?
-The atomic number, located above the chemical symbol on the periodic table, represents the number of protons in the nucleus of an atom. It is unique for each element and is crucial for identifying the element.
How is the atomic mass represented on the periodic table, and what does it signify?
-The atomic mass is usually represented below the chemical symbol and is expressed as a decimal. It indicates the mass of an atom in atomic mass units, factoring in the average mass of the atom's isotopes and their relative abundance.
Why might older chemistry books use the term 'atomic weight' instead of 'atomic mass'?
-Older chemistry books might use 'atomic weight' to refer to what is now commonly called 'atomic mass'. The change in terminology reflects a more accurate understanding of it being a measure of mass rather than weight.
Why is it important for students to have their own periodic table during the tutorial?
-Having a personal copy of the periodic table allows students to directly engage with the tutorial, follow along with explanations, and use the same tool they will rely on during tests, enhancing learning and retention.
What are the key elements of a periodic table that are used most frequently?
-The most frequently used elements of the periodic table include the chemical symbol, atomic number, and atomic mass. These provide essential information about the elements and their properties.
How does understanding the layout of the periodic table benefit students in chemistry?
-Understanding the layout of the periodic table helps students quickly identify and relate the properties of elements, understand trends, and make predictions about chemical behavior, which is fundamental in chemistry studies.
What does the presenter mean by 'we'll go into this in a whole lot more detail later in the course'?
-The presenter indicates that the current discussion on the periodic table is introductory and that more detailed and complex aspects of it will be covered deeper into the course, providing a foundational understanding for future lessons.
How do chemical symbols relate to chemical compounds, as mentioned in the script?
-Chemical symbols are used to represent elements in chemical formulas, such as H2O for water. Understanding these symbols is crucial for discussing and writing chemical compounds accurately.
Outlines
🌟 Introduction to the Periodic Table
Jason introduces the section on the periodic table, emphasizing the importance of understanding its organization. He mentions that they have previously discussed atomic mass and atomic number, which are essential tools for utilizing the periodic table. The goal of this section is to examine the table as a whole and grasp its structure. Jason encourages viewers to have their periodic table at hand, as it's crucial for tests and understanding chemical compounds. He highlights that each element has a chemical symbol in bold, an atomic number above it, and an atomic mass below it. These are the most important elements of the periodic table.
Mindmap
Keywords
💡Periodic Table
💡Chemical Symbol
💡Atomic Number
💡Protons
💡Atomic Mass
💡Chemical Compounds
💡
💡Electron Configuration
💡Nucleus
💡Chemical Properties
💡Atomic Mass Units (amu)
💡Chemistry Textbook
Highlights
Introduction to the periodic table and its importance in chemistry
Elements on the periodic table are organized by atomic mass and atomic number
Explanation of atomic mass and atomic number as listed on the periodic table
Chemical symbols are in bold and are central to each element's identity
Atomic number represents the number of protons in an element's nucleus
Unique atomic number for each element determines its identity
The layout of the periodic table allows for quick identification of proton numbers
Atomic mass is often a decimal and represents the element's mass in atomic mass units
Older chemistry books may refer to atomic mass as atomic weight
Importance of the element symbol, atomic number, and atomic mass for understanding the periodic table
Use of chemical symbols in forming chemical compounds like H2O for water
Encouragement to pull out a physical periodic table for practice, as it's what will be used on tests
Future in-depth exploration of the periodic table in later sections of the course
Cool insights that can be gained from just looking at the layout of the periodic table
The periodic table is a crucial tool for understanding chemical elements and their properties
Elements are arranged in order of increasing atomic number across the table
The periodic table's organization helps in predicting chemical and physical properties of elements
The atomic number sequence reveals the arrangement of elements in the periodic table
Elements with similar properties are grouped together in the same block or family on the periodic table
Understanding the periodic table is fundamental for advanced chemistry studies
Transcripts
Browse More Related Video

Lesson 10 - What is Atomic Mass Of An Element? (Chemistry Tutor)

Atomic Number & Mass Number | Properties of Matter | Chemistry | FuseSchool

Isotope Notation
Periodic Table Explained: Introduction

Isotopes and Isobars | Atoms and Molecules | Don't Memorise

What Is An Atom - Part 1 | Properties of Matter | Chemistry | FuseSchool
5.0 / 5 (0 votes)
Thanks for rating: