Learn about Nuclear Physics, Nuclear Energy, and the Periodic Table of Elements
TLDRThe script delves into the fascinating world of nuclear physics, exploring its origins in the 20th century and its significance beyond controversial applications. It highlights the study of nuclei, their composition, and the forces that hold them together, such as the strong nuclear force. The text also touches on the role of isotopes in nuclear medicine, the table of nuclides, and the processes of nuclear fusion and fission. Furthermore, it explains the concept of half-life and the geological impact of radioactive decay. The script emphasizes the importance of nuclear physics in understanding the universe and the development of valuable technologies, while also shedding light on the fundamental forces of nature and their roles in the behavior of atomic nuclei.
Takeaways
- 🌌 Neutron stars are incredibly dense objects that are essentially giant nuclei.
- ⚗️ The amount of uranium needed to power a large nuclear plant for a year is surprisingly small, fitting under a kitchen table.
- 🏥 Nuclear medicine is increasingly important in modern healthcare, with techniques like PET scans, CAT scans, and proton therapy being widely used.
- 🔬 Nuclear physics emerged in the 20th century from chemistry, focusing on the nucleus and forces much stronger than chemical bonds.
- ☀️ The Sun's energy comes from nuclear fusion, specifically the conversion of hydrogen into helium, a process that cannot be achieved through chemistry alone.
- ⚖️ A nucleus is made of protons and neutrons, held together by the strong nuclear force, which is significantly stronger than the electromagnetic force.
- 🗜️ Nuclei can release energy when split (fission) or when combined (fusion), with the largest nuclei like uranium providing the most energy when split.
- ☢️ Nuclear fission is the energy source behind nuclear bombs and many power plants, while fusion is the principle of hydrogen bombs and potential future clean energy.
- 📊 The table of nuclides organizes over 3,000 isotopes, showing the known nuclei and their stability, with stable isotopes located in a band known as the 'valley of stability'.
- ⏳ The half-life of an isotope is the time it takes for half of it to decay; after 10 half-lives, 99.9% of the original isotope has decayed.
- 🚀 Nuclear reactions can create new isotopes, some of which are highly valuable for medical applications, and can be produced using reactors and accelerators.
Q & A
What is nuclear physics and how did it emerge in the 20th century?
-Nuclear physics is the field of science that studies atomic nuclei and their interactions. It emerged in the 20th century from the realm of chemistry, leading to a deeper understanding of the forces and particles that make up the nucleus of an atom. The discovery of the nucleus by Rutherford in 1911 marked a significant milestone in the development of nuclear physics.
What is the difference between nuclear fission and nuclear fusion?
-Nuclear fission is the process of splitting larger atomic nuclei into smaller ones, such as when uranium nuclei are split to release energy. Nuclear fusion, on the other hand, involves combining smaller nuclei, like hydrogen, to form a larger nucleus, releasing energy in the process. Both processes are powerful sources of energy, but fusion is more challenging for humans to harness.
What is the role of the strong nuclear force in holding the nucleus together?
-The strong nuclear force is a fundamental force that acts between protons and neutrons in the nucleus. It is significantly stronger than the electromagnetic force and is responsible for holding the nucleus together despite the repulsive force between positively charged protons.
What are isotopes and how do they relate to the periodic table?
-Isotopes are variants of a particular chemical element that have the same number of protons but different numbers of neutrons. While the periodic table organizes elements based on the number of protons, each element can have multiple isotopes with varying stability and properties. The table of isotopes expands on this concept, cataloging over 3,000 known isotopes.
How do nuclear physics applications contribute to medical imaging and treatment?
-Nuclear physics applications in medicine include the use of radioactive isotopes for imaging (PET scans, CAT scans) and treatment (proton therapy). These techniques rely on the interactions of nuclear particles with the body's tissues, allowing for the diagnosis and treatment of various conditions, including cancer.
What is the significance of the valley of stability in nuclear physics?
-The valley of stability is a region on the chart of nuclides where nuclei are most stable. Nuclei within this region have a balance of protons and neutrons that minimizes the repulsive electromagnetic force and maintains the stability of the nucleus. Most of the elements found on Earth reside within or near the valley of stability.
How does the process of radioactive decay relate to the geology of our planet?
-Radioactive decay of elements like uranium, thorium, and isotopes of potassium provides heat that keeps Earth's core molten. This molten core is essential for the planet's geological activity, including the movement of tectonic plates and the process of continental drift.
What are the fundamental forces of nature and how do they relate to nuclear physics?
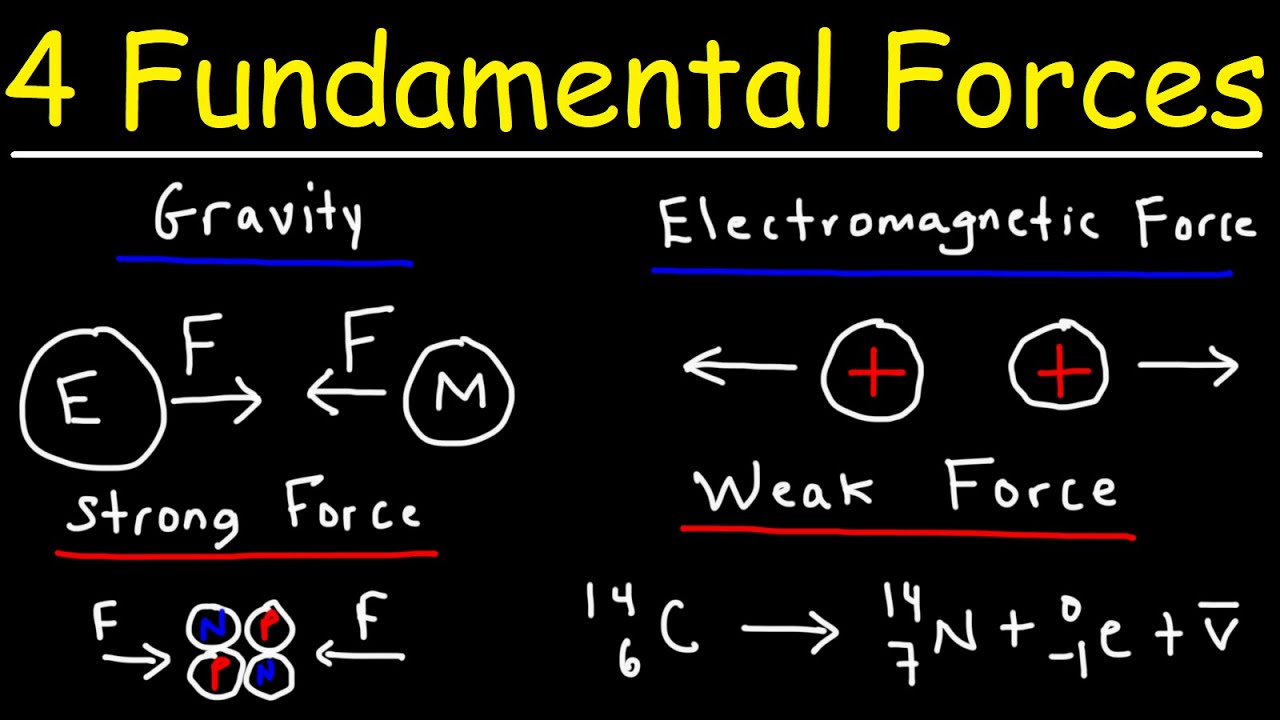
-The fundamental forces of nature include gravity, electromagnetism, the weak nuclear force, and the strong nuclear force. In nuclear physics, the strong nuclear force is crucial for holding the nucleus together, while the weak nuclear force is responsible for processes like beta decay. Electromagnetism plays a role in the repulsion of protons within the nucleus, and gravity, although the weakest force at the atomic scale, affects the structure of neutron stars.
What is the concept of quantum chromodynamics (QCD) in nuclear physics?
-Quantum chromodynamics (QCD) is the theory that describes the strong force and the interactions of quarks and gluons, which are the fundamental constituents of protons and neutrons. QCD explains how quarks are bound together by the exchange of gluons to form the nucleons, and how these nucleons make up the atomic nucleus.
How do scientists study the properties and reactions of atomic nuclei?
-Scientists study atomic nuclei by using particle accelerators to create high-energy collisions between various particles and target nuclei. They then use large particle detectors to measure the outcomes of these collisions, reconstructing the subatomic interactions and gaining insights into the structure and behavior of nuclei.
Outlines
🌠 Introduction to Nuclear Physics
This paragraph introduces the concept of nuclear physics, highlighting its emergence from the realm of chemistry and its significance in understanding the universe. It explains the role of nuclear physics in revealing that the sun is not a chemical fire but a nuclear one, transforming hydrogen into helium. The paragraph also touches on the historical underestimation of the age of the Sun and Earth by Lord Kelvin due to the lack of knowledge in nuclear physics. It further discusses the importance of nuclear physics in various fields such as energy production, nuclear medicine, and the understanding of the periodic table.
📊 Isotopes and Nuclear Stability
The second paragraph delves into the concept of isotopes, which are variants of an element with different numbers of neutrons. It explains how the periodic table organizes atoms based on the number of protons, while the table of isotopes displays over 3,000 known nuclei. The paragraph emphasizes the stability of certain isotopes, which is depicted in the 'valley of stability' on the nuclear chart. It also discusses the process of decay, where unstable isotopes transform over time, and the role of these processes in geological activity and the heat within Earth's core.
🕰️ Understanding Half-Life and Radioactive Decay
This paragraph focuses on the concept of half-life, the time it takes for half of a radioactive element to decay. It provides examples of different isotopes with varying half-lives, from the billions of years for uranium-238 to the mere 6,000 years for carbon-14. The paragraph also explains the notation used in nuclear physics to represent isotopes and the implications of their decay. It further discusses the historical significance of radioactive decay and its applications in nuclear medicine and other scientific fields.
🌌 Nuclear Forces and the Structure of Nuclei
The fourth paragraph discusses the interplay between the strong nuclear force, which holds protons and neutrons together in the nucleus, and the electromagnetic force, which pushes them apart due to the positive charge of protons. It explains how this balance affects the stability and size of nuclei, with lighter nuclei having equal numbers of protons and neutrons and heavier nuclei having more neutrons to counteract the increased repulsion. The paragraph also touches on the concept of the 'valley of stability' and the processes of beta plus and beta minus decay that occur when nuclei have too many protons or neutrons, respectively.
🔬 Studying Nuclei and the Origins of Matter
This paragraph explores how scientists study nuclei through high-energy collisions using particle accelerators. It describes the process of hitting nuclei with various 'hammers' (accelerated particles) and observing the resulting subatomic particles to reconstruct the collisions. The paragraph also discusses the origins of nuclei, from the Big Bang to the stars and supernovae, and the idea that all matter is essentially 'star stuff.' It highlights the importance of nuclear physics in understanding the formation of elements and the potential significance of isotopes no longer present on Earth.
🌐 Applications and Fundamental Forces in Nuclear Physics
The sixth paragraph covers the practical applications of nuclear physics in various fields such as medicine, industry, and archaeology. It explains how the understanding of nuclear physics allows for the development of technologies like X-rays, proton beams, and radioactive isotopes for imaging and treatment. The paragraph also discusses the fundamental forces of nature and their roles in nuclear physics, including gravity, electromagnetism, the weak nuclear force, and the strong nuclear force. It provides insights into how these forces interact with and influence the behavior of nuclei and the broader implications for understanding the universe.
💥 Decay Processes and Quantum Chromodynamics
The final paragraph focuses on the three fundamental types of decay: alpha decay, beta decay, and gamma decay. It explains the conditions under which these decay processes occur and their significance in nuclear transformations. The paragraph also introduces quantum chromodynamics (QCD), the theory that describes the strong force and the interactions between quarks and gluons. It discusses the complex structure of protons and neutrons, the role of the Higgs boson, and the recent advances in applying QCD to understand the subtle changes in the quark structure of nucleons within nuclei.
Mindmap
Keywords
💡Nuclear Physics
💡Nucleus
💡Isotopes
💡Nuclear Force
💡Fission
💡Fusion
💡Periodic Table
💡Radioactivity
💡Half-Life
💡Quantum Chromodynamics
💡Neutron Stars
Highlights
Neutron stars are giant nuclei, showcasing the extreme states of matter possible in the universe.
The amount of uranium fuel needed for a year of nuclear power plant operation is surprisingly small, fitting under a kitchen table.
Nuclear medicine techniques like PET scans, CAT scans, and proton therapy are increasingly relied upon by doctors.
Nuclear physics emerged in the 20th century, revealing the sun's energy source and correcting previous misconceptions about the age of the Earth.
Nuclei combine and take apart through nuclear fusion and fission, releasing vast amounts of energy.
Iron has the greatest binding energy, making it the hardest nucleus to split and a prime candidate for nuclear energy release.
Hydrogen nuclei fusion is the process occurring in the Sun, providing energy for life on Earth.
Nuclear fission is the energy source behind nuclear bombs and power plants, while fusion powers hydrogen bombs.
Scientists are working on harnessing fusion energy for clean, unlimited power by fusing hydrogen isotopes into helium.
The periodic table organizes atoms by proton count, but isotopes vary in neutron numbers, leading to different properties.
The table of isotopes maps over 3,000 known nuclei, with new ones continually discovered, and shows the stability of different isotopes.
Nuclear physics is not just about energy production; it's also about studying the fundamental properties and behaviors of atomic nuclei.
Nuclear reactions can transmute elements, as seen in the historical quest to turn lead into gold, which is now possible with particle accelerators.
Nuclear physics explores the origins of elements, from the Big Bang to the life cycles of stars and the creation of heavy elements in supernovae.
The study of nuclei involves using particle accelerators and detectors to recreate and analyze subatomic collisions, leading to new insights and technologies.
Nuclear physics utilizes all four fundamental forces of nature, with gravity, electromagnetism, weak nuclear force, and strong nuclear force all playing roles in the behavior of atomic nuclei.
The strong nuclear force, arising from quantum chromodynamics, is responsible for holding protons and neutrons together within the nucleus.
Nuclear decay processes, such as alpha, beta, and gamma decay, involve changes in the nucleus and are fundamental to understanding radioactivity and dating geological features.
The study of nuclear physics has broad applications, from medical imaging and treatment to industrial analysis and understanding the universe's history.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: