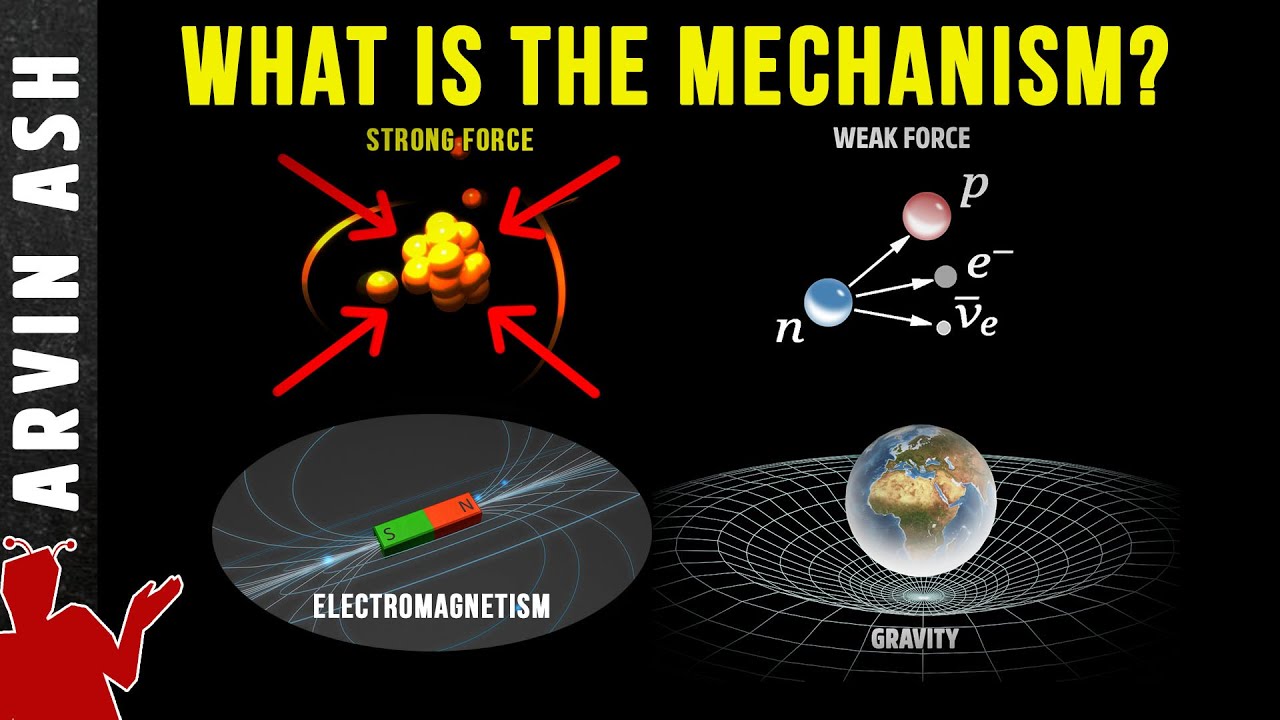
The Four Fundamental Forces of Nature
TLDRThis lesson delves into the four fundamental forces in nature: gravity, electromagnetism, the strong nuclear force, and the weak nuclear force. Gravity, a long-range force, attracts large objects like planets and moons. Electromagnetism, also long-range, involves both electric and magnetic forces, acting on charges and creating fields. The strong nuclear force, a short-range force, holds atomic nuclei together despite electromagnetic repulsion. Lastly, the weak nuclear force governs radioactive decay, exemplified by beta decay in carbon-14. Each force plays a critical role in shaping our universe.
Takeaways
- 🌍 Gravity or the gravitational force becomes important when dealing with very large objects like planets and stars.
- 🌕 Gravity is a force of attraction, not repulsion, and its strength increases with the mass of the objects involved.
- 📏 The gravitational force decreases greatly with increased distance between two objects.
- 🔋 The electromagnetic force can act over long distances and involves both electric and magnetic forces.
- ➕➖ Like charges repel each other, while opposite charges attract, demonstrating the nature of electric forces.
- 🧲 Moving charges, such as electrons in a wire, create magnetic fields around them.
- 💥 The strong nuclear force is a short-range force that holds the nucleus of an atom together, overcoming the repulsive electromagnetic force between protons.
- 🔬 The strong nuclear force keeps protons and neutrons intact within the nucleus, ensuring the stability of atoms.
- ☢️ The weak nuclear force is associated with radioactive decay and processes like beta decay.
- 🔄 In beta decay, a neutron in carbon-14 turns into a proton, emitting a beta particle (electron) and an anti-neutrino, transforming the element into nitrogen-14.
Q & A
What are the four fundamental forces found in nature?
-The four fundamental forces found in nature are gravity (gravitational force), electromagnetic force, strong nuclear force, and weak nuclear force.
How does gravity act on large objects such as planets and the Sun?
-Gravity acts as a force of attraction between large objects. It can be calculated using the equation G * (M1 * M2) / r^2, where G is the gravitational constant, M1 and M2 are the masses of the objects, and r is the distance between them.
What is the relationship between the masses of two planets and the gravitational force between them?
-The gravitational force between two planets increases as the masses of the planets increase. This is due to the direct proportionality between mass and gravitational force in the gravitational equation.
How does the distance between two objects affect the gravitational force?
-The gravitational force decreases greatly as the distance between two objects increases. This is because the force is inversely proportional to the square of the distance (r^2) in the gravitational equation.
What is the range of the gravitational force?
-Gravity is a long-range force that can act over very large distances, such as the distance between the Earth and the Moon, which is approximately 238,900 miles.
What is the electromagnetic force and how does it differ from gravity?
-The electromagnetic force is a long-range force that acts between charged particles, causing them to attract or repel each other based on their charges. Unlike gravity, which is always attractive, the electromagnetic force can be either attractive or repulsive.
How do electric charges interact with each other in terms of the electromagnetic force?
-Like charges repel each other, while opposite charges attract each other. This interaction is governed by the electric component of the electromagnetic force.
What is the strong nuclear force and how does it differ from the electromagnetic force?
-The strong nuclear force is a short-range force that acts within the nucleus of an atom, holding protons and neutrons together despite the electromagnetic repulsion between the positively charged protons. It is much stronger than the electromagnetic force at very short distances.
Why does the strong nuclear force keep protons together in an atomic nucleus?
-The strong nuclear force overcomes the repulsive electromagnetic force between protons, binding them together within the nucleus and maintaining the stability of the nucleus.
What is the weak nuclear force and how is it related to radioactive decay?
-The weak nuclear force is responsible for certain types of radioactive decay, such as beta decay. It is involved in processes where one element transforms into another, as seen in the decay of carbon-14 into nitrogen-14, accompanied by the emission of a beta particle (electron) and an anti-neutrino.
How does the weak nuclear force differ from the other fundamental forces?
-The weak nuclear force is unique in that it is associated with nuclear reactions and radioactive decay, unlike the other forces which have broader applications in the physical world.
Outlines
🌌 Fundamental Forces of Nature: Gravity and Electromagnetism
This paragraph introduces the four fundamental forces found in nature, starting with gravity. It explains gravity as a force of attraction between large objects like planets and the Sun, and describes its calculation using the equation G * (M1 * M2) / r^2, where G is the gravitational constant, M1 and M2 are the masses of the objects, and r is the distance between them. The paragraph also discusses how increasing mass or decreasing distance increases gravitational force. It then transitions to the electromagnetic force, which includes both electric and magnetic forces. The electric force is described in terms of repulsion between like charges and attraction between opposite charges. The creation of magnetic fields by moving charges, such as electrons in a wire, is also highlighted, showing the relationship between electric and magnetic fields.
🔬 Nuclear Forces: Strong and Weak Interactions
The second paragraph delves into the strong nuclear force, which is a short-range force that holds the protons and neutrons together in an atomic nucleus, overcoming the repulsive electromagnetic force between protons. The stability of the helium nucleus, with its two protons and two neutrons, is used as an example to illustrate this force. The weak nuclear force is then introduced as it relates to radioactive decay, specifically beta decay. The paragraph provides the example of carbon-14 decaying into nitrogen-14 by emitting a beta particle (electron) and an anti-neutrino, thereby changing the element. The weak nuclear force is crucial in such nuclear reactions and radioactive decay processes.
Mindmap
Keywords
💡Gravitational Force
💡Electromagnetic Force
💡Strong Nuclear Force
💡Weak Nuclear Force
💡Nucleus
💡Beta Decay
💡Charge
💡Magnetic Field
💡Electric Field
💡Proton
💡Neutron
Highlights
Introduction to the four fundamental forces found in nature.
Gravity, the gravitational force, is important for very large objects like Earth, Sun, and Moon.
Gravity is a force of attraction, not repulsion.
The gravitational force can be calculated using the equation G * (M1 * M2) / r^2.
Increasing mass of planets increases the gravitational force they can exert.
Increasing distance between objects decreases the gravitational force.
Gravity is a long-range force that can act over large distances.
The distance between Earth and Moon is 238,900 miles where gravity acts.
Introduction to the electromagnetic force, which is also a long-range force.
Electric force causes repulsion between two positive charges and attraction between opposite charges.
Magnetic force is demonstrated by the repulsion between two North Poles of bar magnets.
A moving charge can create its own magnetic field.
Electric and magnetic fields are related and can be created by charged particles.
Introduction to the strong nuclear force, a short-range force.
The strong nuclear force holds protons and neutrons together in an atomic nucleus.
The strong nuclear force overcomes the repulsion of electromagnetic force in the nucleus.
Introduction to the weak nuclear force, associated with radioactive decay.
Beta decay in carbon-14 as an example of a process involving the weak nuclear force.
The weak nuclear force is responsible for nuclear reactions and radioactive decay.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: