Predicting Products | Synthesis Reactions
TLDRThe video script introduces the concept of synthesis reactions, emphasizing the process of combining elements to form compounds. It explains that synthesis involves two elements reacting to create a single product, and provides a method for predicting these products by 'criss-crossing' the charges of the elements. The script uses examples like sodium chloride, aluminum fluoride, magnesium chloride, and beryllium oxide to illustrate the prediction process, encouraging viewers to practice predicting products for other synthesis reactions.
Takeaways
- 🧪 A synthesis reaction involves combining elements or simpler compounds to form a more complex product.
- 🔬 Recognize synthesis reactions by identifying two elements as reactants, indicating a formation of a new compound.
- 🏗️ Start with simple synthesis reactions, typically involving one element reacting with another to produce a compound.
- 🌟 Be aware of diatomic elements like chlorine (Cl2), which are considered single elements in reactions.
- 🔄 To predict products, combine the elements from the reactants into a single compound.
- ✂️ 'Criscross' the charges of the elements to determine the formula of the product compound.
- 📈 Use the periodic table to identify the charges of elements based on their group numbers.
- 🔢 For example, Group 1 elements have a +1 charge, and Group 17 elements have a -1 charge.
- 🤹♂️ Practice predicting products by applying the crisscross method to a series of given reactions.
- 📝 Remember that the initial focus is on predicting products, not balancing the chemical equations.
- 🎓 Take time to practice with additional problems to solidify understanding of synthesis reactions and product prediction.
Q & A
What is the main topic of the video?
-The main topic of the video is how to predict products for synthesis reactions.
What does the term 'synthesis' mean in the context of chemistry?
-In the context of chemistry, 'synthesis' means to make or create, referring to the process of combining reactants to form a single product.
How can you recognize a synthesis reaction just from the reactants?
-You can recognize a synthesis reaction from the reactants if they are two elements that combine to form a single compound.
What is a diatomic element, and how does it relate to synthesis reactions?
-A diatomic element is an element that naturally exists as a pair of atoms bonded together, like chlorine in Cl2. It relates to synthesis reactions as it still counts as a single element in reactions, even though it is a compound in form.
How do you predict the product of a synthesis reaction involving aluminum and fluorine?
-To predict the product of a synthesis reaction involving aluminum and fluorine, you combine the two elements into a single compound and criss-cross their charges (aluminum with +3 and fluorine with -1) to get AlF3.
What is the general rule for predicting the product of a synthesis reaction with elements from groups 1 to 18?
-The general rule for predicting the product of a synthesis reaction is to combine the elements into a single compound and criss-cross their charges based on their group numbers in the periodic table (+1 for group 1, +2 for group 2, -1 for group 17, -2 for group 16, -3 for group 15, and +3 for aluminum).
How do you predict the product when magnesium reacts with chlorine gas?
-When magnesium reacts with chlorine gas, the product is a compound containing magnesium and chlorine. By criss-crossing the charges (magnesium with +2 and chlorine with -1), you get MgCl2.
What happens when elements with equal and opposite charges react in a synthesis reaction?
-When elements with equal and opposite charges react in a synthesis reaction, they cancel each other out, leaving just the combination of the two elements as the product (for example, CaO from calcium and oxygen).
How do you predict the product when silver reacts with sulfur?
-When silver reacts with sulfur, the product is a compound consisting of silver and sulfur. By criss-crossing the charges (silver with +1 and sulfur with -2), the final product is Ag2S.
What is the significance of balancing in synthesis reactions, and why isn't it covered in this video?
-Balancing in synthesis reactions is significant because it ensures the conservation of mass and charge. However, this video doesn't cover balancing as it focuses only on predicting the products without going into the details of reaction stoichiometry.
Outlines
🧪 Understanding Synthesis Reactions
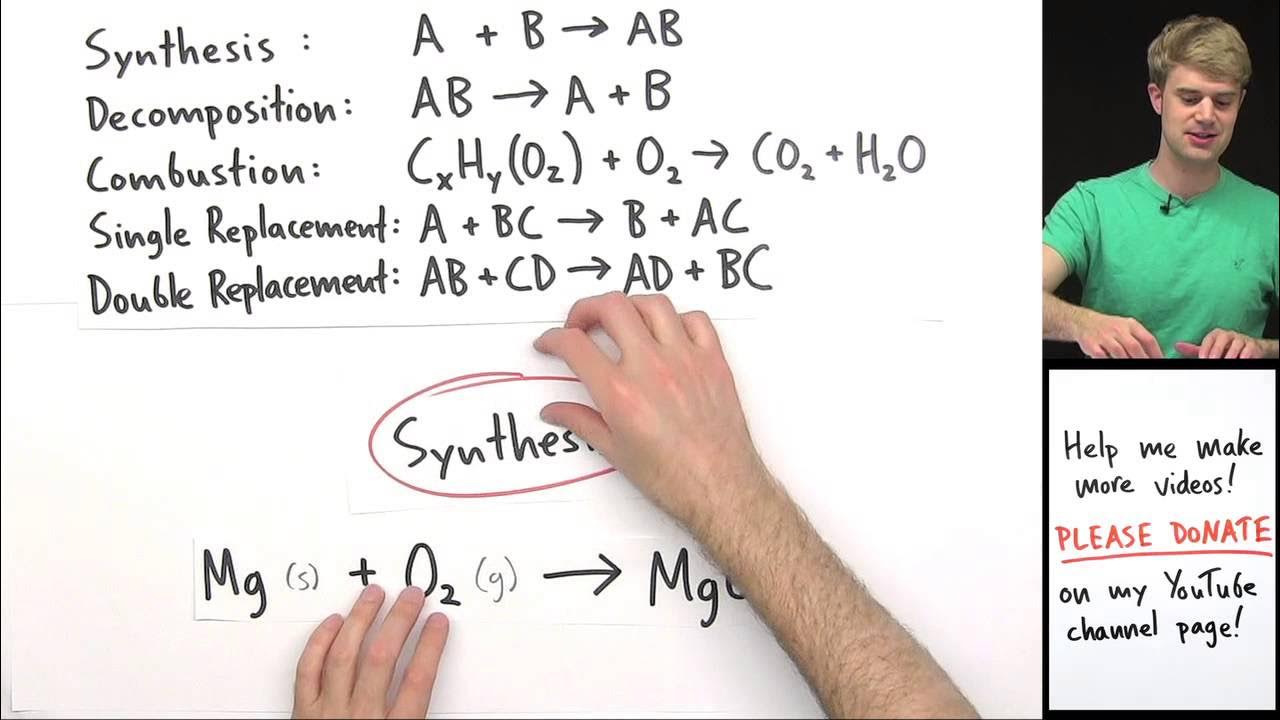
This paragraph introduces the concept of synthesis reactions, explaining that they involve combining elements or simpler compounds to form a more complex product. It emphasizes recognizing synthesis reactions by identifying two elements as reactants and highlights the importance of knowing diatomic elements, such as chlorine (Cl2), which are still considered elements in these reactions. The paragraph also outlines the process of predicting products by 'criss-crossing' the charges of the reacting elements to determine the formula of the resulting compound, using examples like aluminum and fluorine forming AlF3 and magnesium and chlorine forming MgCl2.
📚 Predicting Products for Synthesis Reactions
This paragraph delves deeper into the process of predicting products for synthesis reactions. It explains how to identify the charges of elements based on their position in the periodic table and how to use this information to predict the outcome of reactions. The paragraph provides examples of predicting products for reactions involving elements like zinc and iodine, calcium and oxygen, sodium and bromine, and silver and sulfur. It reinforces the concept of criss-crossing charges to determine the formula of the product, such as ZnI2 for zinc and iodine, CaO for calcium and oxygen, NaBr for sodium and bromine, and Ag2S for silver and sulfur.
Mindmap
Keywords
💡Synthesis Reaction
💡Reactants
💡Products
💡Elements
💡Compounds
💡Diatomic Elements
💡Charges
💡Periodic Table
💡Balancing Chemical Equations
💡Predicting Products
💡Practice Problems
Highlights
The video discusses predicting products for synthesis reactions, a key concept in chemistry.
Synthesis reactions involve combining elements or simpler compounds to form a more complex product.
The term 'synthesis' refers to the creation or formation of a new entity from existing parts.
Identifying synthesis reactions involves recognizing when two elements are reactants.
Diatomic elements, like chlorine (Cl2), are considered single elements in reactions, not compounds.
An example of a synthesis reaction is the combination of sodium (Na) and chlorine (Cl) to form sodium chloride (NaCl).
The process of predicting synthesis reaction products involves combining elements into a single compound.
When predicting products, it's essential to 'criss-cross' the charges of the elements to determine the compound's formula.
The video provides a method for predicting products without balancing the equations, focusing solely on the product side.
An example given is the reaction between aluminum and fluorine, resulting in the compound AlF3.
The charges of elements in a compound are derived from their group numbers in the periodic table.
The video demonstrates predicting the product of magnesium reacting with chlorine gas, resulting in MgCl2.
Beneath the surface, the video teaches fundamental chemistry concepts like oxidation states and compound nomenclature.
The product of beryllium and oxygen gas is predicted to be a compound containing BeO, following the criss-cross rule.
Silver and sulfur reacting together form a compound, with the video illustrating the criss-cross method for predicting Ag2S.
The video concludes with practice problems to reinforce the method of predicting synthesis reaction products.
Transcripts
Browse More Related Video

Predicting Products | Decomposition Reactions

Chemical Reaction (5 of 11) Synthesis Reactions, an Explanation

Solving Chemical Reactions - Predicting the Products - CLEAR & SIMPLE CHEMISTRY
Classifying Types of Chemical Reactions Practice Problems

Synthesis Reactions: Predict the Products (100 Examples)

Predicting Products of Chemical Reactions: Practice Problems
5.0 / 5 (0 votes)
Thanks for rating: