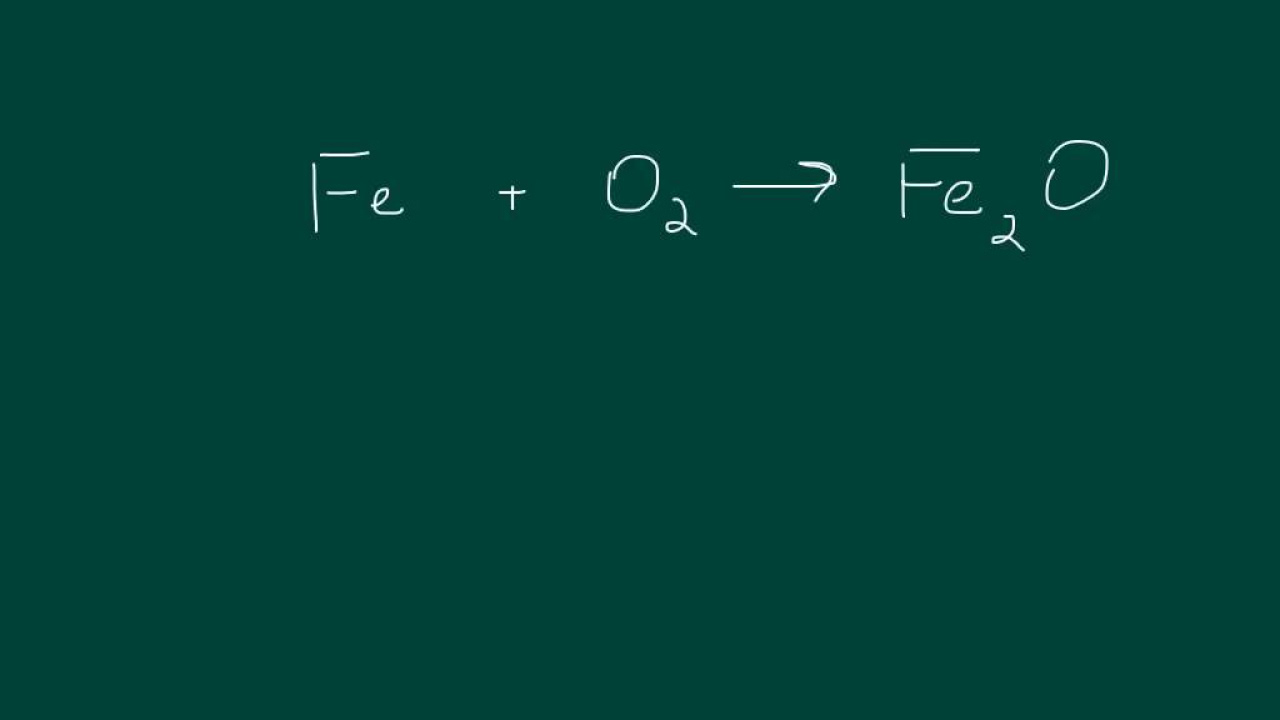Chemical Reaction (5 of 11) Synthesis Reactions, an Explanation
TLDRThis video script offers a comprehensive overview of synthesis reactions, detailing how two or more substances chemically bond to create a single, new compound. It categorizes synthesis reactions into three types: elements combining, compounds reacting, and a compound with an element. The script provides examples of each, demonstrating how to balance chemical equations using the crossover rule and coefficients. The video also visually shows the synthesis of iron oxide, silver chloride, magnesium oxide, and zinc sulfide, emphasizing the exothermic nature of these reactions and the formation of a single product compound.
Takeaways
- 🧪 Synthesis reactions involve two or more substances combining to form a single, new compound.
- 🔬 The hallmark of a synthesis reaction is one product on the right-hand side of the chemical equation.
- 🌐 There are three types of synthesis reactions: two elements combining, two compounds reacting, and a compound with an element.
- 🔥 Examples include carbon and oxygen forming CO2, calcium oxide and water forming Ca(OH)2, and CO and O2 forming CO2.
- 📈 The reaction between iron and oxygen results in iron oxide (Fe2O3), commonly known as rust.
- 🔢 To balance chemical equations, use the crossover rule to determine the ratio of elements and ion charges.
- 💡 Silver metal reacts with chlorine gas to form silver chloride (AgCl), with a 1:1 ratio of silver to chloride.
- 🌟 Magnesium metal reacts with oxygen to form magnesium oxide (MgO), a white powder with a bright white characteristic color.
- ⚗️ Zinc metal reacts with sulfur to form zinc sulfide (ZnS), an exothermic reaction releasing energy due to bond formation.
- 🌡️ Synthesis reactions are typically exothermic, as energy is released when new chemical bonds are made.
Q & A
What is a synthesis reaction?
-A synthesis reaction is a chemical process where two or more substances react to form a single, new substance.
How can you identify a synthesis reaction?
-You can identify a synthesis reaction by looking for a single product on the right-hand side of the chemical equation, with reactants (elements or compounds) on the left-hand side.
What are the three types of synthesis reactions mentioned in the script?
-The three types of synthesis reactions are: 1) Two elements combining, such as carbon and oxygen forming carbon dioxide; 2) Two compounds reacting, like calcium oxide and water producing calcium hydroxide; 3) A compound and an element reacting, such as carbon monoxide and oxygen gas forming carbon dioxide.
What is the product of the reaction between iron and oxygen?
-The product of the reaction between iron and oxygen is a compound containing both iron and oxygen, which is Fe2O3, commonly known as rust or iron oxide.
How do you balance the chemical equation for the reaction between iron and oxygen?
-To balance the equation for the reaction between iron and oxygen, you use the crossover method, resulting in 4 Fe (iron) and 3 O2 (oxygen) on the reactant side, and 2 Fe2O3 (iron(III) oxide) on the product side.
What is the product of the reaction between silver metal and chlorine gas?
-The product of the reaction between silver metal and chlorine gas is silver chloride (AgCl), with a 1:1 ratio of silver to chloride in the compound.
What is the balanced chemical equation for the synthesis reaction between magnesium and oxygen?
-The balanced chemical equation for the reaction between magnesium and oxygen is 2 Mg + O2 → 2 MgO, resulting in magnesium oxide.
What is the characteristic color of the product formed in the synthesis reaction between magnesium and oxygen?
-The product, magnesium oxide, is a white powder and exhibits a bright white color.
What is the product of the synthesis reaction between zinc metal and sulfur?
-The product of the synthesis reaction between zinc metal and sulfur is zinc sulfide (ZnS).
What charge do zinc and sulfur typically form in compounds?
-Zinc typically forms a +2 charge and sulfur forms a -2 charge in compounds.
Why are synthesis reactions usually exothermic?
-Synthesis reactions are usually exothermic because they involve the formation of chemical bonds, which releases energy.
Outlines
📚 Introduction to Synthesis Reactions
This paragraph introduces synthesis reactions, explaining that they occur when two or more substances react to form a single new substance. The key to identifying a synthesis reaction is the presence of a single product on the right-hand side of the chemical equation. The reactants on the left-hand side can be elements or compounds. The video discusses three types of synthesis reactions: two elements combining, two compounds reacting, and a compound with an element. The common factor in all these reactions is the formation of a single compound as the product. Examples are provided, such as the synthesis of iron oxide (rust), silver chloride, and magnesium oxide, with a detailed explanation of how to balance the chemical equations using the crossover method.
🔬 Experiments and Observations of Synthesis Reactions
The second paragraph focuses on experimental demonstrations and observations of synthesis reactions. It describes the process of reacting zinc with sulfur, showing the exothermic nature of the reaction and the formation of zinc sulfide. The video also explains the charges of the elements involved in the reactions and how these charges help determine the stoichiometric ratios in the balanced chemical equations. The summary emphasizes the general characteristics of synthesis reactions, such as being exothermic and resulting in the formation of a single compound product.
Mindmap
Keywords
💡Synthesis reactions
💡Chemical bond
💡Single product
💡Elements and compounds
💡Charge
💡Crossover rule
💡Exothermic reactions
💡Balancing chemical equations
💡Reactants
💡Products
💡Chemical equation
Highlights
Synthesis reactions involve two or more substances chemically bonding to form a single, new substance.
The key to identifying a synthesis reaction is having a single product on the right-hand side of the chemical equation.
In a synthesis reaction, reactants can be elements or compounds, but the product is always a single compound.
There are three different kinds of synthesis reactions: two elements combining, two compounds reacting, and a compound reacting with an element.
An example of two elements combining is carbon and oxygen forming carbon dioxide.
An example of two compounds reacting is calcium oxide and water producing calcium hydroxide.
An example of a compound and an element reacting is carbon monoxide and oxygen gas forming carbon dioxide.
All synthesis reactions have a single product, which is always a compound.
The synthesis reaction between iron and oxygen results in the compound iron oxide, commonly known as rust.
Balancing the chemical equation for iron and oxygen involves using the crossover rule to determine the ratio of iron to oxygen.
Silver metal reacts with chlorine gas to form a single compound, silver chloride, with a 1:1 ratio of silver to chloride.
The synthesis reaction between magnesium metal and oxygen produces magnesium oxide, which is a white powder.
Magnesium and oxygen have a 1:1 ratio in the formation of magnesium oxide, with two oxygens on both the reactant and product sides.
The synthesis reaction between zinc metal and sulfur results in the formation of zinc sulfide through an exothermic reaction.
Zinc and sulfur form a 1:1 ratio in zinc sulfide, with zinc having a +2 charge and sulfur a -2 charge.
Synthesis reactions are typically exothermic as they involve the formation of chemical bonds and the release of energy.
The single product in a synthesis reaction is always a compound, signifying the essence of these reactions.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: