Applied Chemistry_ Hydrogen Spectrum_ Lecture 04 for Polytechnic 1st Semester
TLDRThe video script discusses the process of printing a hydrogen spectrum using code and its relation to the model of the atom. It explains the concept of electrons transitioning between energy levels and the release of energy in the form of light. The script also touches on the importance of subscribing to the channel and sharing the class with friends. The explanation includes the use of electric energy to excite the hydrogen gas sample and the resulting spectral lines that can be observed when the energy supply is stopped. The content is engaging, informative, and encourages viewers to learn more about the principles behind the hydrogen spectrum and atomic model.
Takeaways
- 🔬 The script discusses the process of printing a hydrogen spectrum using code and relates it to the model of the atom.
- 🌟 The video emphasizes the importance of subscribing to the channel and sharing the class with friends to not forget the content.
- 📚 It explains the concept of electrons transitioning between energy levels and the release of energy in the form of light.
- 💡 The role of electric energy supply is highlighted in causing the electrons to transition and the resulting emission of a spectrum.
- 🚀 The script touches upon the concept of 'attempts to electrons' and how they get animated and move to a side after absorbing energy.
- 🌈 The hydrogen spectrum is explained as a series of lines, each representing the release of energy at different wavelengths.
- 📈 The video uses the example of a container with hydrogen gas and the supply of electric energy to demonstrate the electron transitions.
- 🔄 The process of electrons moving from a higher energy orbit to a lower one and the associated energy release is detailed.
- 📊 The script also delves into the concept of 'virtual' cases and how they might affect the energy levels and transitions.
- 🔢 Numerical examples are provided to illustrate the calculations involved in understanding the energy changes and transitions.
- 🎓 The video concludes with a brief mention of the upcoming topics, including the hydrogenburg uncertainty principle and quantum numbers.
Q & A
What is the main topic discussed in the script?
-The main topic discussed in the script is the process of printing the hydrogen spectrum by understanding the energy levels of electrons and their transitions in a hydrogen atom.
What does the script suggest at the beginning for new viewers?
-The script suggests that new viewers should subscribe to the channel and share the class with their friends.
How does the script describe the process of electrons moving to a lower energy level?
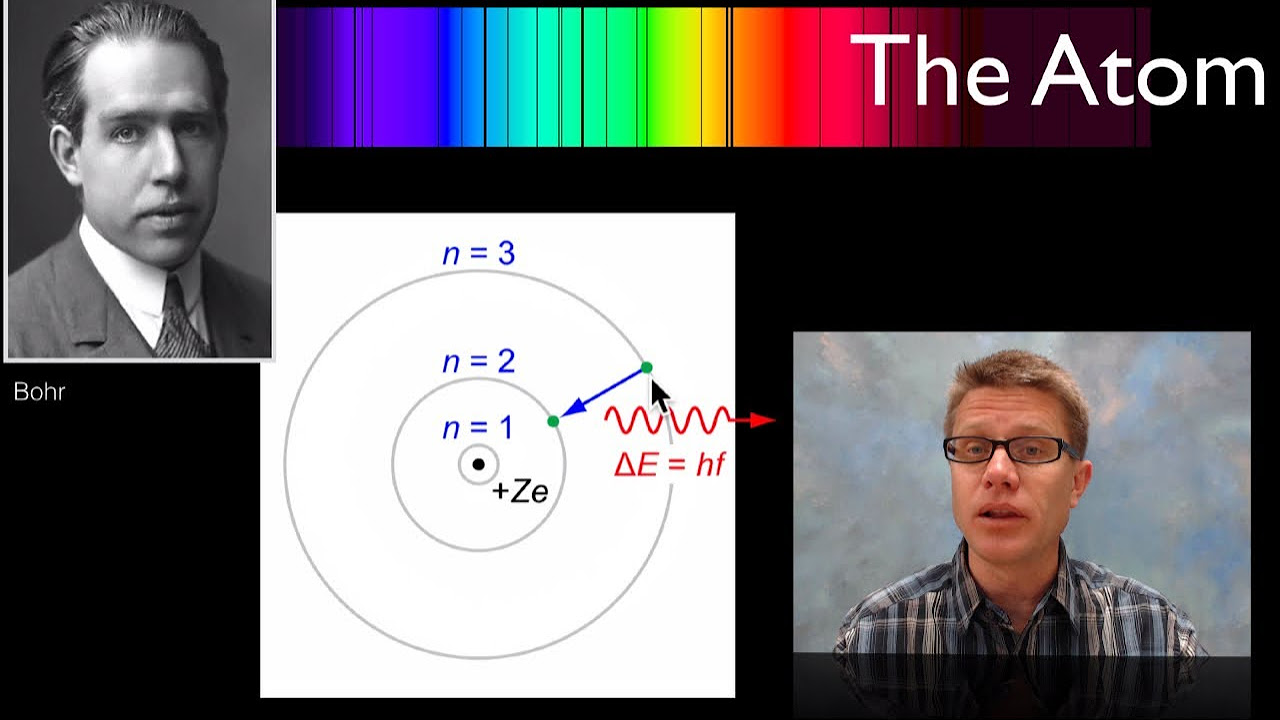
-The script describes that when electrons move from a higher energy level to a lower one, they release energy in the form of photons, which can be observed as spectral lines.
What is the significance of the energy supplied to the hydrogen sample in the script?
-The energy supplied to the hydrogen sample excites the electrons to higher energy levels. When these electrons return to their original or lower energy levels, they release energy in the form of light, creating the hydrogen spectrum.
What is the role of electric energy supply mentioned in the script?
-The electric energy supply is used to excite the electrons in the hydrogen atoms, causing them to move to higher energy levels. This is a crucial step in the process of creating the hydrogen spectrum.
What does the script mean by 'exited state' of an electron?
-The 'exited state' refers to the condition when an electron has absorbed energy and moved to a higher energy level. Once in the exited state, the electron is unstable and will eventually return to a lower energy level, emitting a photon in the process.
How does the script explain the concept of energy levels and electron transitions?
-The script explains that electrons in an atom exist in specific energy levels. When an electron transitions from one level to another, it either absorbs or releases energy corresponding to the difference in energy between the levels. This energy difference is manifested as photons of specific frequencies, which can be observed as spectral lines.
What is the importance of understanding the hydrogen spectrum as described in the script?
-Understanding the hydrogen spectrum is important as it provides insights into the behavior of electrons in atoms, their energy levels, and the interactions of light with matter. It also has practical applications in fields like astrophysics, spectroscopy, and quantum mechanics.
What is the role of订阅 (subscription) in the context of the script?
-In the context of the script, subscribing (订阅) refers to following the channel to receive updates on new content. It is emphasized as a way to support the channel and ensure that viewers do not miss out on future educational content.
What is the term used in the script to describe the release of energy when an electron returns to a lower energy level?
-The term used in the script to describe the release of energy when an electron returns to a lower energy level is 'energy release' or 'release of energy'.
How does the script relate the concept of energy levels to the production of spectral lines?
-The script relates the concept of energy levels to the production of spectral lines by explaining that the difference in energy between levels corresponds to the energy of the photons emitted when an electron transitions. These photons have specific frequencies that appear as distinct lines in a spectrum.
Outlines
🚀 Introduction to Hydrogen Spectrum Printing
The paragraph introduces the topic of printing the hydrogen spectrum using code into votes to make a model. The speaker, identified as Arke Singh, welcomes the audience to the products and explains the process of starting the class. He emphasizes the importance of subscribing to the channel and sharing the class with friends. The paragraph discusses the creation of a model using the board and learning about electronic conditions. It also touches on the concept of applying an external item's image, explaining the electronic channel and attempts to electrons, leading to excitation and emission of energy. The speaker uses the example of a tip providing energy, causing electrons to emit and become excited, eventually leading to a change in the item's state. The paragraph concludes with a discussion on the potential outcomes if the channel's subscription is canceled, relating it to the excited condition of the model.
🌟 Explanation of Hydrogen Spectrum
This paragraph delves deeper into understanding the hydrogen spectrum by providing an explanation. The speaker describes a scenario where a sample of hydrogen gas is taken and subjected to an electric supply, causing the hydrogen atoms within the sample to have their electrons excited to different energy levels. The paragraph explains the process of electrons absorbing energy and moving to different orbits, and then releasing energy in the form of light when they return to a stable condition. The speaker also discusses the concept of a particular term's value, relating it to the energy levels of hydrogen atoms and their electrons. The explanation includes the idea of an electron residing in the first orbit and the energy it would release if it were to move to a third orbit. The paragraph concludes with a discussion on the energy formula and the change in energy that would be observed.
🔬 Observation of Electrons and Energy Release
The speaker continues the discussion on electrons and their behavior when energy is supplied and then removed. The paragraph describes the process of electrons attempting to achieve a stable condition and releasing energy in the process. It talks about the electrons creating an image of the energy they release and then leaving it behind to return to a lower energy orbit. The speaker uses the analogy of a person selling something to explain the possible outcomes of electrons moving to different orbits and releasing varying amounts of energy. The paragraph also touches on the concept of different energy subscriptions and the resulting shifts in electron behavior. The discussion concludes with a look at what happens when the supply of electric energy is stopped, and the electrons attempt to use the remaining energy to release more energy.
🌈 Visualizing the Hydrogen Spectrum
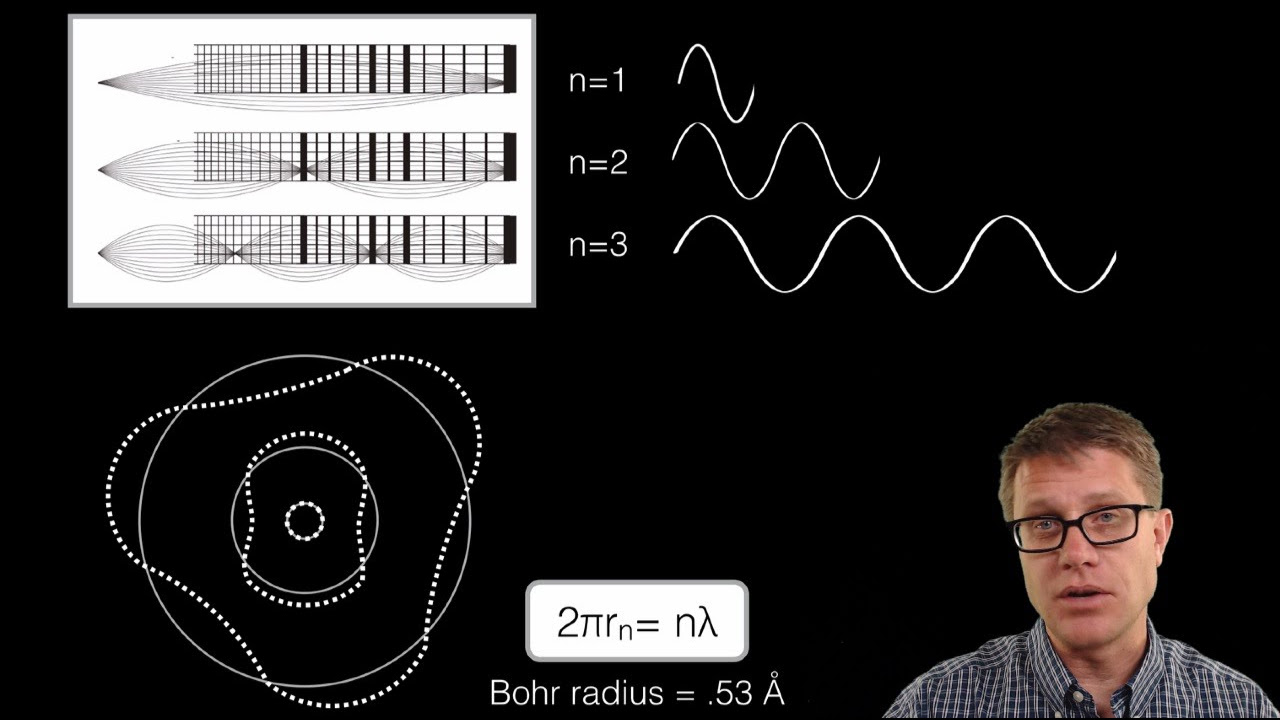
In this paragraph, the speaker talks about visualizing the hydrogen spectrum by imagining the behavior of electrons in a sample of hydrogen gas. The speaker describes the process of electrons being excited to different energy levels and then releasing energy in the form of light as they return to a lower energy state. The paragraph discusses the concept of different energy levels and the corresponding wavelengths of light that are emitted. It also talks about the idea of a series of lines that represent the different energy transitions of the hydrogen atoms. The speaker uses the example of a prism to explain how the different wavelengths of light can be separated and observed. The paragraph concludes with a discussion on the potential for different electrons to be in different orbits and the resulting series of lines that would be observed in the spectrum.
📚 Quantum Numbers and Atomic Model Calculations
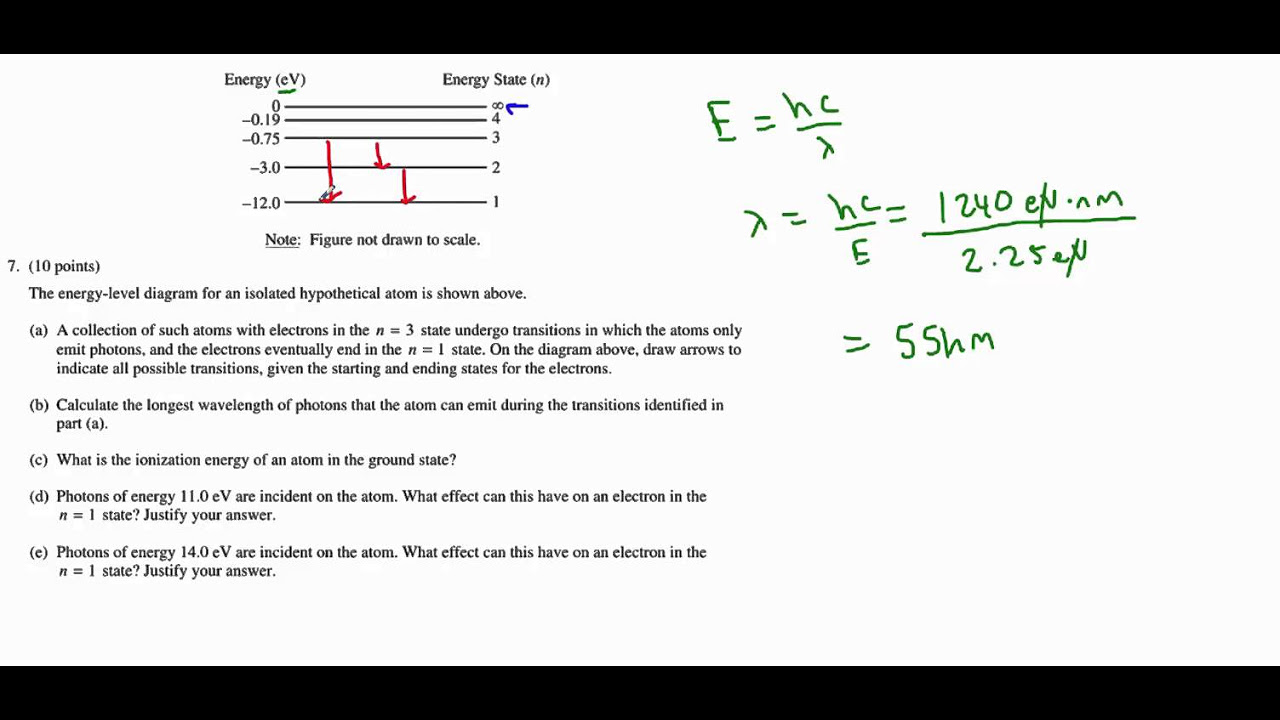
The speaker discusses the concept of quantum numbers and their role in understanding the behavior of electrons in an atom. The paragraph explains the Bohr atomic model and the energy levels associated with it. The speaker provides calculations related to the first and second orbits of an electron and the energy they possess. The paragraph also touches on the concept of electron volts and the energy released when an electron transitions from one orbit to another. The speaker uses the example of an electron transitioning from the first orbit to a higher energy level and the resulting change in energy. The paragraph concludes with a discussion on the energy associated with different orbits and the potential for electrons to exist in a state of zero energy.
🎓 Conclusion and Future Lessons
The speaker concludes the lesson on the hydrogen spectrum and introduces the topic of the Hertzberg uncertainty principle to be discussed in future classes. The paragraph emphasizes the importance of understanding quantum numbers and their application in calculating the energy levels of atoms. The speaker encourages the audience to subscribe to the channel for more lessons and to share the class with friends. The paragraph concludes with a reminder to like the video and subscribe to the channel for more educational content.
Mindmap
Keywords
💡Hydrogen Spectrum
💡Energy Levels
💡Quantum Mechanics
💡Electron Transitions
💡Spectral Lines
💡Electric Energy
💡Orbital
💡Energy Absorption and Release
💡Stabilization
💡Quantum Numbers
💡Electric Field
Highlights
The introduction of the hydrogen spectrum and its importance in understanding atomic structure.
Exploring the process of printing the hydrogen spectrum code into votes to make a model.
The significance of understanding the energy levels of electrons in the hydrogen atom.
The concept of excited states and how electrons transition between these states.
The role of energy supply in the excitation of electrons and their subsequent return to a stable state.
The explanation of the electric field and its effect on the electron's energy levels.
The discussion on the emission of light when energy is supplied to the hydrogen sample.
The process of stopping the supply of electric energy and observing the electron's behavior.
The concept of different energy subscriptions and how they affect the electron's orbit.
The explanation of the spectrum's appearance when observing the hydrogen atom's electrons in an excited state.
The scientific possibility of an electron transitioning from one orbit to another.
The description of the energy release formula and its calculation.
The practical application of understanding the hydrogen spectrum in technology and atomic models.
The importance of subscribing to the channel for more informative content on topics like the hydrogen spectrum.
The encouragement for viewers to share the class with friends and engage in learning together.
The mention of quantum numbers and their role in understanding the electron's position in a stable condition.
The conclusion of the class with an encouragement to learn about the hydrogenburg uncertainty principle in future classes.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: