Speed of Light, Frequency, and Wavelength Calculations - Chemistry Practice Problems
TLDRThis educational video script explores the relationship between the wavelength and frequency of photons, using the equation c = λν to demonstrate calculations. It explains how to find the wavelength given frequency, and vice versa, converting between different units like meters, micrometers, and nanometers. The script also covers how frequency and wavelength are inversely related and introduces Planck's constant to calculate frequency from photon energy, concluding with determining photon wavelength from energy, all presented in a clear and engaging manner.
Takeaways
- 🔬 The video discusses problems related to finding the wavelength and frequency of photons.
- 📏 The speed of light (c) is 3 x 10^8 meters per second.
- 📉 Wavelength (λ) is calculated by dividing the speed of light by the frequency (ν).
- 📡 A photon with a frequency of 2.5 x 10^12 Hz has a wavelength of 1.2 x 10^-4 meters, which converts to 120 micrometers.
- 🔢 The frequency of a photon with a wavelength of 1.5 x 10^-8 meters is 2 x 10^16 Hz.
- 🔍 To convert nanometers to meters, replace nanometers with 10^-9 meters.
- 📐 A photon with a wavelength of 350 nanometers has a frequency of 8.57 x 10^14 Hz.
- 📡 A photon with a frequency of 95 megahertz has a wavelength of 3.16 meters.
- 🔄 Wavelength and frequency are inversely related; as one increases, the other decreases.
- 🔋 The energy of a photon is related to its frequency through Planck's constant (h = 6.626 x 10^-34 J·s).
Q & A
What is the formula used to calculate the wavelength of a photon given its frequency?
-The formula used is c = λ * ν, where c is the speed of light, λ represents the wavelength in meters, and ν is the frequency in hertz.
What is the speed of light in meters per second?
-The speed of light is 3 times 10 to the 8 meters per second.
How do you convert the wavelength from meters to micrometers?
-Since one micrometer is 10 to the negative six meters, you multiply the value in meters by 10 to the positive six to convert it to micrometers.
What is the frequency of a photon with a wavelength of 1.5 times 10 to the negative 8 meters?
-The frequency is calculated by dividing the speed of light by the wavelength, resulting in 2 times 10 to the 16 hertz.
How do you convert nanometers to meters for the purpose of calculating photon frequency?
-You replace nanometers with 10 to the minus 9 meters to convert it to the appropriate unit for the calculation.
What is the relationship between the wavelength and frequency of a photon?
-The wavelength and frequency of a photon are inversely related, meaning as one increases, the other decreases.
What is Planck's constant and what is its value?
-Planck's constant, represented by the symbol h, is a fundamental constant in quantum mechanics with a value of 6.626 times 10 to the negative 34 joules times seconds.
How do you calculate the frequency of a photon given its energy?
-The frequency is calculated by dividing the energy of the photon by Planck's constant.
If the energy of a photon is 4.3 times 10 to the negative 19 joules, what is its frequency?
-The frequency is 6.49 times 10 to the 14 hertz, obtained by dividing the given energy by Planck's constant.
What is the wavelength of a photon with a frequency of 95 megahertz?
-The wavelength is 3.16 meters, calculated by dividing the speed of light by the frequency after converting megahertz to hertz.
How do you convert the wavelength from meters to nanometers?
-Since one nanometer is 10 to the minus 9 meters, you multiply the value in meters by 10 to the positive 9 to convert it to nanometers.
Outlines
🔬 Calculating Photon Wavelength and Frequency
This paragraph introduces the video's focus on calculating the wavelength and frequency of photons. It explains using the equation c = λν, where c is the speed of light, λ is the wavelength, and ν is the frequency. The example given calculates the wavelength of a photon with a frequency of 2.5 x 10^12 Hz, resulting in 120 micrometers after converting from meters. The paragraph also touches on the potential need for unit conversions in such problems.
🌌 Frequency Calculation from Wavelength and Vice Versa
The second paragraph delves into the inverse relationship between wavelength and frequency of photons, using the formula ν = c/λ. It provides examples of calculating the frequency from a given wavelength and vice versa, emphasizing the constant speed of light in a vacuum. The summary includes the calculation of the frequency for a photon with a wavelength of 1.5 x 10^-8 meters, resulting in 2 x 10^16 Hz, and the frequency of a photon with a wavelength of 350 nanometers, yielding 8.57 x 10^14 Hz after converting nanometers to meters.
🚀 Photon Wavelength and Frequency from Energy
The final paragraph discusses how to determine the wavelength and frequency of a photon when given its energy. It introduces Planck's constant (h = 6.626 x 10^-34 Js) and the equation E = hν to relate photon energy to frequency. The summary includes the calculation of the frequency for a photon with an energy of 3.5 x 10^-18 J, resulting in 5.28 x 10^15 Hz, and subsequently the wavelength, which is 4.62 x 10^-7 meters or 462 nanometers after conversion.
Mindmap
Keywords
💡Wavelength
💡Frequency
💡Photon
💡Speed of Light
💡Hertz
💡Micrometers
💡Planck's Constant
💡Energy of a Photon
💡Nanometers
💡Inversely Related
Highlights
The video focuses on calculating the wavelength and frequency of a photon.
The fundamental equation for wavelength and frequency is c = λν, where c is the speed of light, λ is the wavelength, and ν is the frequency.
The wavelength is calculated by dividing the speed of light by the frequency.
The speed of light is a constant 3 x 10^8 meters per second.
Frequency is expressed in hertz, which can also be written as seconds to the power of -1.
Conversion from meters to micrometers is achieved by adjusting the exponent from -6 to +6.
The frequency of a photon can be found by dividing the speed of light by its wavelength.
Wavelengths are sometimes given in nanometers, which require conversion to meters for calculations.
The speed of light remains constant in a vacuum, but it changes when passing through different materials.
The relationship between wavelength and frequency is inversely proportional.
The energy of a photon is calculated using Planck's constant, h = 6.626 x 10^-34 joules seconds.
Frequency can be calculated from the energy of a photon by dividing the energy by Planck's constant.
The video provides a step-by-step guide to convert megahertz to hertz for wavelength calculations.
An example calculation is given to determine the wavelength of a photon with a frequency of 95 megahertz.
The video concludes with a problem-solving example for determining the wavelength of a photon with a given energy.
Conversion from meters to nanometers is explained for finalizing the calculation of photon wavelength.
The video emphasizes the importance of unit conversion in photon calculations.
The final answer for the photon wavelength in nanometers is provided.
Transcripts
Browse More Related Video
Electromagnetic Spectrum - Basic Introduction
Visible Light Spectrum Explained - Wavelength Range / Color Chart Diagram - Chemistry

Electromagnetic Spectrum Explained - Gamma X rays Microwaves Infrared Radio Waves UV Visble Light

Wave Speed Practice Problems v2
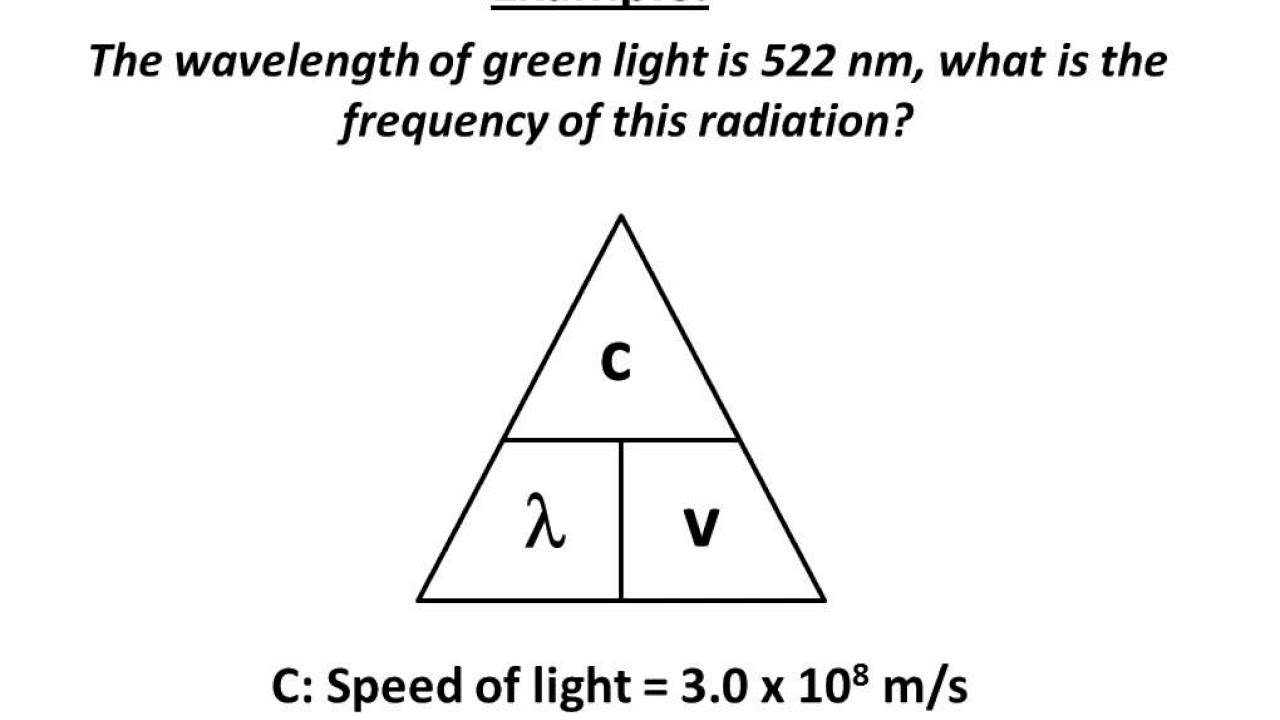
Wavelength-Frequency equation

How to Find the Wavelength, Frequency, Energy and Photons | Study Chemistry With Us
5.0 / 5 (0 votes)
Thanks for rating: