Study Designs (Cross-sectional, Case-control, Cohort) | Statistics Tutorial | MarinStatsLectures
TLDRThis video script delves into various study designs for data collection, contrasting observational and experimental approaches. It highlights the strengths and limitations of each, from the descriptive case studies to the more controlled randomized control trials. The focus is on understanding how these designs contribute to the evidence hierarchy, with an example of smoking and lung cancer to illustrate the differences. The script emphasizes the importance of temporality, the ability to estimate prevalence and incidence, and the challenge of confounding variables in observational studies, while noting the ethical considerations and practicalities of experimental designs.
Takeaways
- 📊 Observational and experimental studies are the two main categories of data collection in health research.
- 🔍 Observational studies involve watching and recording data without intervening, commonly used in health research.
- 🧪 Experimental studies attempt to control or manipulate variables, such as randomizing treatments.
- 📝 The evidence provided by study designs ranges from weakest to strongest, with case studies at the weakest end.
- 👤 Case studies or series are descriptive analyses of one or a few individuals, often used for rare or new diseases.
- 🌐 Descriptive studies describe features of a population, useful for formulating hypotheses about potential causes.
- 🏞 Ecological studies analyze data for entire groups, but suffer from the ecological fallacy, ignoring individual differences.
- 📸 Cross-sectional studies provide a snapshot in time, useful for estimating prevalence but lack temporality.
- 🧬 Case-control designs select individuals based on outcomes, good for rare diseases but cannot estimate incidence or prevalence.
- 👣 Cohort studies follow individuals over time based on exposure, providing temporality and quality records but can be costly and time-consuming.
- 🎯 Randomized control trials balance out confounders by randomly assigning exposures, but may have ethical and practical limitations.
Q & A
What are the two main categories of study designs used for collecting data?
-The two main categories of study designs are observational and experimental studies.
What is the primary difference between observational and experimental studies?
-Observational studies involve observing people without intervening or controlling the situation, whereas experimental studies attempt to control or manipulate variables.
Why are most health research studies observational?
-Most health research studies are observational because they involve monitoring people living their lives and recording data without the need for intervention, which is often more feasible and ethical in health-related research.
What is a case study or case series?
-A case study or case series is a descriptive or exploratory analysis of one or a few individuals, often used to understand rare or newer diseases and discover potential causes.
How does an ecological study differ from a case study?
-An ecological study uses an entire group of people as a single unit for analysis, examining aggregated data for the group, whereas a case study focuses on detailed observations of individual cases.
What is the main limitation of ecological studies?
-The main limitation of ecological studies is the ecological fallacy, where inferences about individuals are based on aggregated statistics of a group, ignoring individual differences.
What are the advantages and disadvantages of cross-sectional studies?
-Advantages of cross-sectional studies include being relatively quick and inexpensive to conduct. However, they lack temporality, meaning it's unclear which event occurred first, and they cannot estimate incidence or prevalence accurately.
What is a case-control study and how does it address the issue of rare diseases?
-A case-control study selects individuals based on the outcome (cases with the disease and controls without it) and examines their exposure to risk factors. It is useful for studying rare diseases because it allows researchers to gather data without waiting a long time for outcomes to occur.
What are the benefits of cohort studies compared to case-control studies?
-Cohort studies provide temporality by following individuals over time, allowing for the recording of changes in exposure and more reliable data. They can also measure incidence rates of diseases.
What are the challenges of cohort studies?
-Cohort studies are challenging due to their long duration, high cost, and potential for loss to follow-up, which can introduce bias in the results.
How do randomized controlled trials differ from observational studies?
-Randomized controlled trials involve randomly assigning participants to different exposure or treatment groups, which helps balance out confounding factors and eliminates concerns about confounding variables.
What ethical considerations are there for randomized controlled trials in the context of smoking and lung cancer?
-Randomized controlled trials in this context would be unethical because they would involve intentionally exposing individuals to a harmful substance (smoking) to observe the effects on lung cancer.
Outlines
🔍 Introduction to Study Designs
This paragraph introduces the two main categories of study designs: observational and experimental. It emphasizes that health research often relies on observational studies, which involve observing people without intervention. The paragraph also outlines the focus on observational study designs and uses the example of studying the link between smoking and lung cancer to illustrate different study designs. The order of these designs is arranged from weakest to strongest evidence, starting with case studies and case series, which are descriptive analyses of individuals or small groups, often used for rare or newer diseases.
📊 Understanding Observational Study Types
This section delves into various types of observational studies, starting with descriptive studies that aim to describe population features, similar to case studies but on a larger scale. It then discusses ecological studies, which analyze entire populations as single units, highlighting the issue of ecological fallacy. Cross-sectional studies are described as snapshots in time, useful for estimating prevalence but lacking temporality. Case-control designs are explained as comparisons between people with and without an outcome, useful for rare diseases but with limitations in temporality and recall. Finally, cohort studies are introduced as longitudinal studies that follow people over time based on exposure, providing temporality and quality records but are time-consuming and expensive.
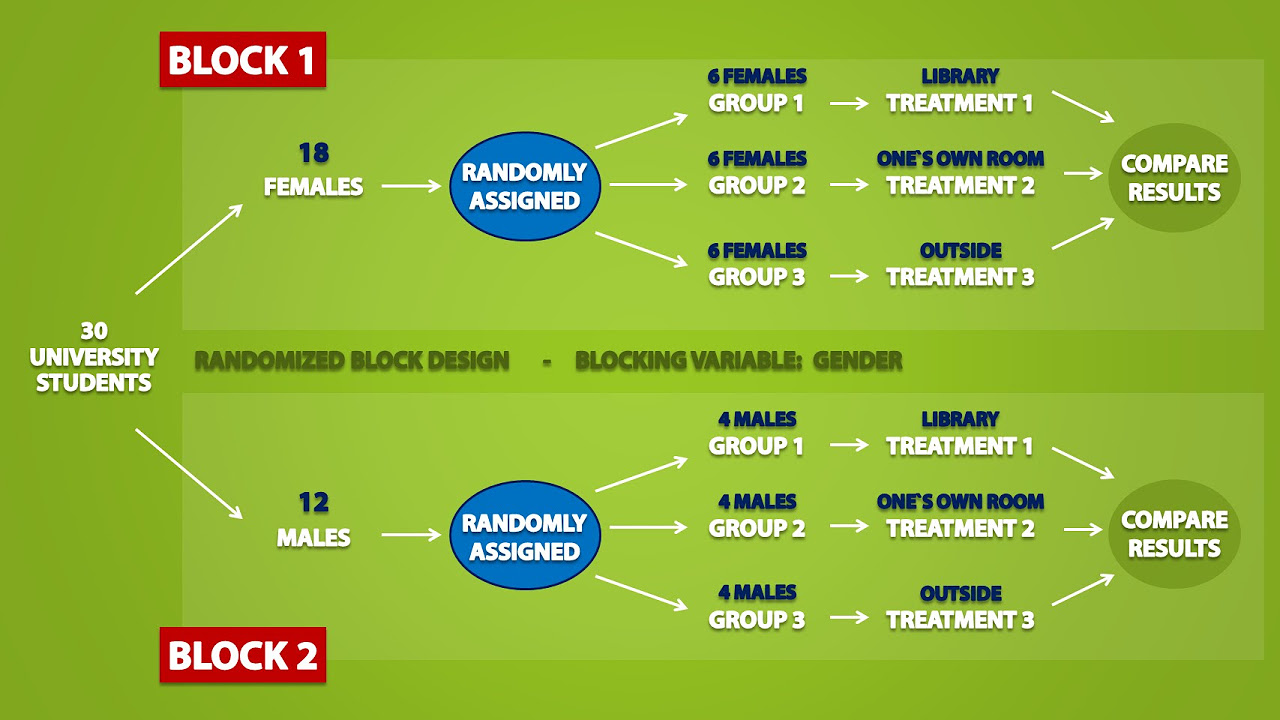
🧪 Randomized Control Trials and Ethical Considerations
This paragraph contrasts randomized control trials with cohort studies, emphasizing the random assignment of exposures or treatments, which helps balance out confounders. Although unethical in the context of smoking and lung cancer, the example serves to illustrate the advantages of experiments in controlling for confounding factors. The paragraph concludes with a brief mention of prospective and retrospective cohort studies, and the challenges of observational studies, such as self-selection and confounding. It wraps up with a reminder of the ethical considerations and the non-invasive nature of observational studies compared to experimental designs.
Mindmap
Keywords
💡Observational Studies
💡Experimental Studies
💡Confounding
💡Temporality
💡Prevalence
💡Incidence
💡Randomization
💡Ecological Fallacy
💡Case-Control Design
💡Cohort Design
💡Prospective and Retrospective Cohorts
Highlights
Studies are categorized into observational and experimental, with health research often focusing on observational studies.
Observational studies involve observing people without intervening or controlling the situation, such as monitoring individuals' lifestyles and recording data.
Experimental studies attempt to control or manipulate variables, like randomizing which treatment an individual receives.
The video will focus on observational study designs, using the example of smoking and lung cancer to explore different designs.
Case studies or case series involve a descriptive analysis of one or a few individuals, often used for rare or newer diseases.
Descriptive studies aim to describe features of a population, noting patterns such as a correlation between smoking and lung cancer rates.
Ecological studies analyze data for entire groups, like countries, but suffer from the ecological fallacy, ignoring individual differences.
Cross-sectional studies provide a snapshot in time, useful for estimating prevalence but lack temporality and can be affected by recall bias.
Case-control designs select people based on the outcome and are good for studying rare diseases and can be quicker and cheaper than cohort studies.
Cohort studies follow people over time based on exposure, providing temporality and quality records, but can be expensive and time-consuming.
Randomized control trials balance out confounders by randomly assigning exposures, but can be unethical and costly.
Observational studies face challenges with confounding, where self-selection of exposure (like smoking) leads to differences beyond the variable of interest.
Case-control studies can be more effective than cohort studies when dealing with rare diseases due to the low expected incidence in a cohort.
Cohort studies can be prospective, following people forward in time, or retrospective, looking back in time for exposure before following participants.
The video provides an overview of different study designs, essential for understanding how data is collected and analyzed in health research.
The hierarchy of study designs ranges from the weakest evidence (case studies) to the strongest (randomized control trials), depending on the context.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: