How Small Is An Atom? Spoiler: Very Small.
TLDRThe video script explores the minuscule scale of atoms, using analogies like stacking carbon atoms to the thickness of a human hair or comparing the size of an atom to the vastness of the Earth. It delves into the composition of atoms, highlighting the roles of protons, neutrons, and electrons, and the concept of empty space filled with quantum fluctuations. The script also touches on the idea of fundamental particles and the simplicity of elemental building blocks, emphasizing the ongoing evolution of our understanding of atoms and the universe.
Takeaways
- 🌟 Atoms are incredibly small, with a single human hair being about as thick as 500,000 carbon atoms stacked together.
- 🔍 A fist-sized object contains trillions of atoms, and if an atom were marble-sized, the fist would be about the size of Earth.
- 👀 The scale of atoms is difficult to imagine, but comparing the tip of a little finger to a room and filling it with rice grains helps visualize the size of cells and proteins.
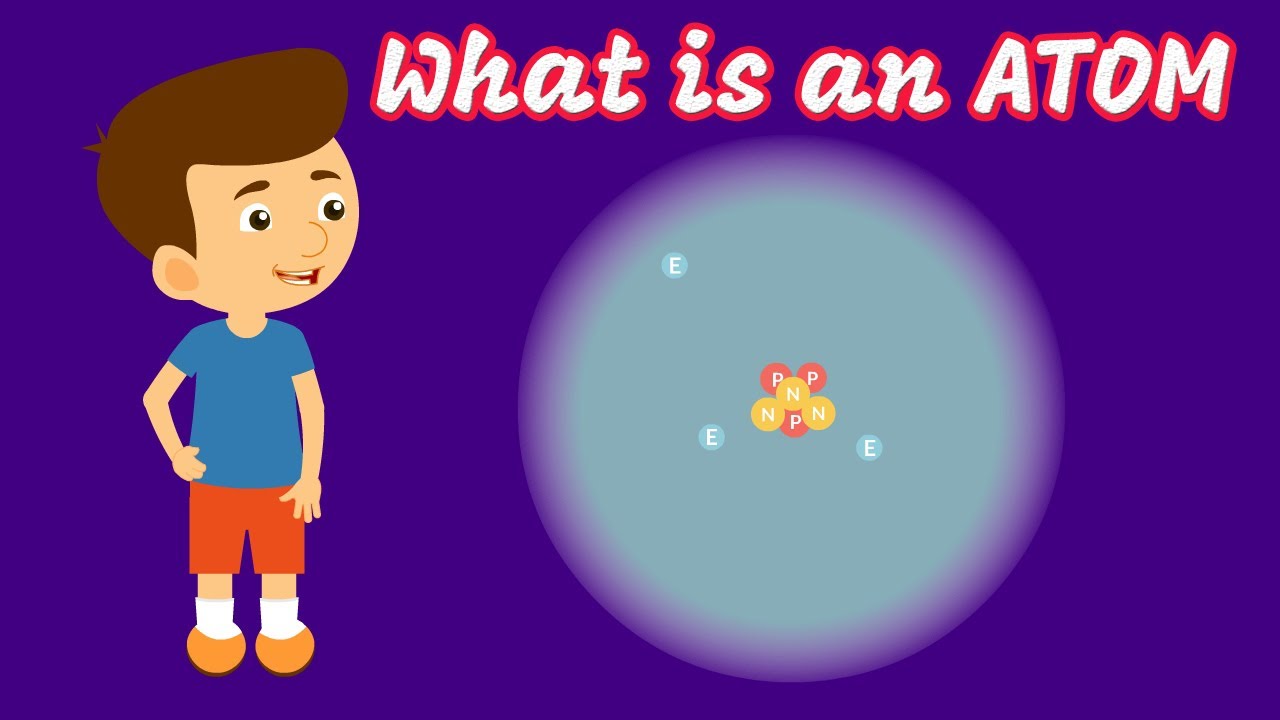
- 🤔 Atoms consist of protons, neutrons, and electrons – the protons and neutrons form the core, while electrons orbit around it.
- 🧪 Protons and neutrons are made up of quarks, which are held together by gluons and are thought to be zero-dimensional particles.
- ⚡ Electrons orbit the atom core at a speed of approximately 2,200 km/s, which is fast enough to circle the Earth in just over 18 seconds.
- 🌌 99.999999999999% of an atom's volume is empty space, but this emptiness is filled with quantum fluctuations and potential energy fields.
- 🏗️ If all the spaces within atoms were removed, the Empire State Building would shrink to the size of a rice grain.
- 🌠 All atoms of a given element are identical, and the hydrogen in your body is the same as the hydrogen in the Sun.
- 🤯 The universe operates on scales and principles that are beyond our everyday comprehension, and our understanding of atoms and their components continues to evolve.
- 💡 Supporting scientific research is crucial for uncovering more about the fundamental nature of the universe and the particles that make up everything.
Q & A
How small are atoms in comparison to a human hair?
-Atoms are so small that a single human hair is about as thick as 500,000 carbon atoms stacked on top of each other.
What does a fist-sized object contain if each atom was as big as a marble?
-If each atom was as big as a marble, a fist-sized object would be about the size of Earth.
What is the significance of comparing a fingertip's tip to the size of a room?
-The comparison helps to visualize the vast number of atoms in a small area, as filling the room with grains of rice represents the cells in just the fingertip's tip.
How big is a protein compared to the room-sized fingertip cell?
-A protein would be as big as the room you're in if the fingertip cell was room-sized.
What are the three elementary particles that make up an atom?
-An atom consists of protons, neutrons, and electrons.
What fundamental force holds protons and neutrons together in an atom's core?
-Protons and neutrons are held together by the strong interaction, one of the four fundamental forces in the universe.
What are quarks and how are they thought to be structured?
-Quarks are particles that make up protons and neutrons, and they are thought to be zero-dimensional points, held together by gluons.
How fast do electrons orbit the atom core?
-Electrons orbit the atom core at a speed of about 2,200 km/s, which is fast enough to get around the Earth in just over 18 seconds.
What fills the empty space in an atom?
-The perceived emptiness in an atom is actually filled by quantum fluctuations, fields with potential energy that build and dissolve spontaneously.
How much space do the atom cores and electrons actually occupy?
-If all the spaces between atom cores were removed, the Empire State Building would be about the size of a grain of rice.
How many atoms of humanity could fit in a teaspoon?
-All the atoms of humanity could fit in a teaspoon.
What is the composition of hydrogen and helium atoms?
-Hydrogen is composed of one proton and one electron, while helium is composed of two protons, two neutrons, and electrons.
How are electrons represented in the context of quantum mechanics?
-Electrons are represented as wave functions and particles at the same time, with clouds of probability called orbitals indicating where they might be with 95% certainty.
Outlines
🤯 The Astonishing Smallness of Atoms
This paragraph introduces the mind-boggling concept of atomic scale. It begins by highlighting the minuscule size of atoms, using the analogy of a human hair's thickness compared to 500,000 carbon atoms. The paragraph then invites the viewer to imagine the scale of atoms by comparing a marble-sized atom to the size of Earth. It proceeds to describe a thought experiment involving the magnification of a fingertip's tip to the size of a room, and further zooming in to the scale of a protein and atoms. The paragraph explains that atoms consist of protons, neutrons, and electrons, with a focus on their fundamental nature and the space they occupy. It touches on the concept of quantum fluctuations filling the perceived emptiness within atoms. The paragraph concludes by emphasizing the vast emptiness within atoms and the extreme compactness of matter in neutron stars. It also mentions the simplicity of atomic composition, with only three elementary particles making up all known elements, and the universality of these elements across the cosmos.
Mindmap
Keywords
💡Atoms
💡Quantum Fluctuations
💡Elementary Particles
💡Quarks
💡Electrons
💡Strong Interaction
💡Empirical Scale
💡Neutron Star
💡Orbitals
💡Quantum Mechanics
💡Fundamental Forces
Highlights
Atoms are incredibly small, with a single human hair being as thick as 500,000 carbon atoms stacked together.
A fist-sized object contains trillions of atoms, illustrating the vast scale of atomic structures.
If an atom were the size of a marble, the entire Earth could fit into the volume of a human fist.
The tip of a little finger, when scaled up to the size of a room, represents the scale of a single cell.
A room filled with grains of rice, each representing a cell, gives an idea of the size of a protein.
Atoms consist of three elementary particles: neutrons, protons, and electrons.
Protons and neutrons are made from quarks and gluons, which are held together by the strong interaction.
Quarks might be zero-dimensional points, representing the most fundamental components of matter.
Electrons orbit the atom core at a speed of about 2,200 km/s, taking just over 18 seconds to orbit the Earth.
99.999999999999% of an atom's volume is empty space, filled with quantum fluctuations and potential energy.
The core and electrons of an atom would be as big as a rice corn if the Empire State Building were compressed to that scale.
All atoms of a given element in the universe are identical, such as all hydrogen atoms being the same.
Electrons exist as both wave functions and particles, with their location described by probability clouds called orbitals.
The electron of an atom could theoretically be on the other side of the universe, as the probability never reaches zero.
The universe is made up of matter composed of only three types of elementary particles for dozens of known elements.
Our understanding of atoms has evolved over time, and the current model is not expected to be the final one.
Support for scientific research is crucial for uncovering more about the strange and fascinating world of atomic and subatomic particles.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: