What is Energy & Work in Chemistry & Physics? - [1-1-6]
TLDRThis lesson delves into the nature of energy, focusing on potential, kinetic, and work as fundamental concepts. The instructor uses relatable examples like rubber bands and gravity to explain potential energy as the propensity to do work. Kinetic energy is introduced as the energy of motion, with work being the energy used when a force moves an object. The lesson emphasizes the conservation of energy, illustrating how potential energy is converted into kinetic or work. The content is crucial for understanding chemistry and physics, as it reveals the driving forces behind chemical reactions and the universe's tendency to transition from high to low energy states.
Takeaways
- 💡 The lesson focuses on the fundamental concepts of potential energy, kinetic energy, and work, emphasizing their significance in understanding physics and chemistry.
- 📢 Emphasizes the importance of discussing energy concepts early in chemistry education to better understand molecular behavior and chemical reactions.
- 🔬 Potential energy is described as the energy stored within an object due to its position or condition, using a stretched rubber band as a relatable example.
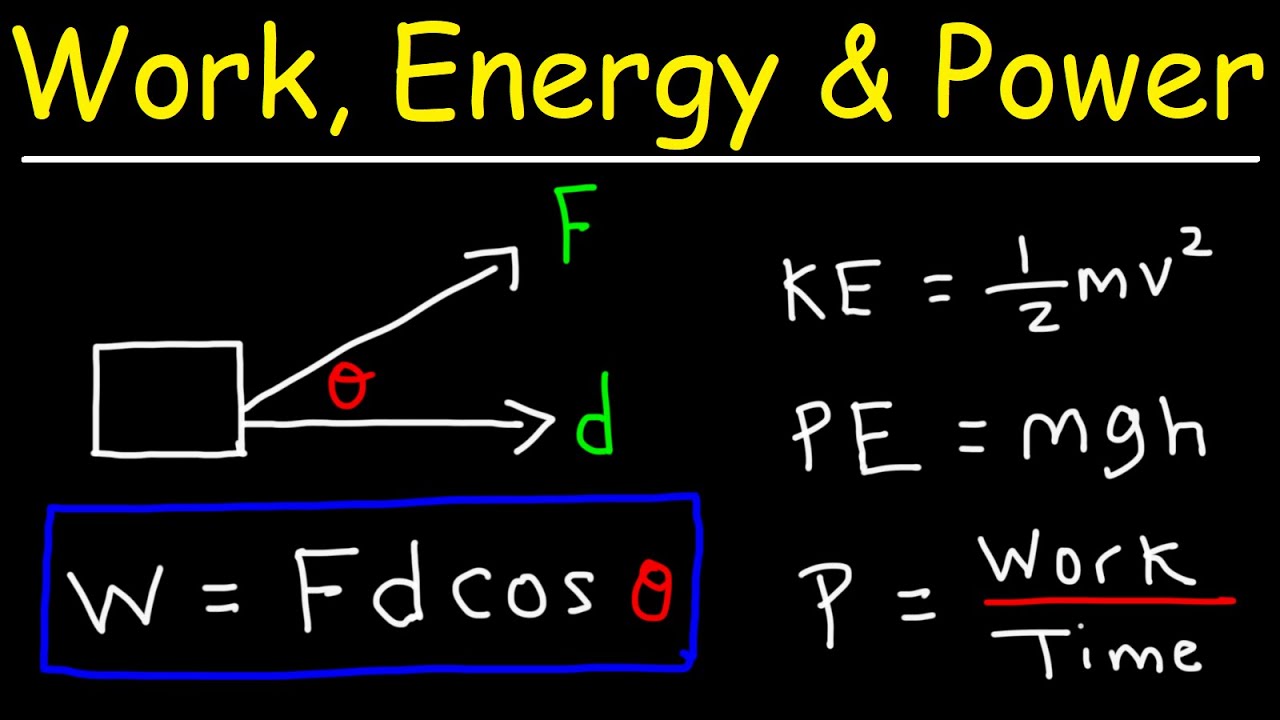
- 🤖 Work is defined as the energy used when a force moves an object through a distance, highlighting the relationship between force, distance, and energy.
- 🚀 Kinetic energy is presented as the energy of motion, emphasizing its dependence on an object's mass and velocity.
- 📈 The concept of conservation of energy is underscored, illustrating how energy transitions between potential, kinetic, and work without being lost.
- 💧 Uses everyday examples, such as a roller coaster, to illustrate the interplay between potential energy and kinetic energy.
- 🗯 Explores how the principles of energy dynamics underpin chemical reactions, with nature tending to move towards lower energy states for stability.
- 🛠 Draws parallels between gravitational and electric forces to bridge understanding of macroscopic phenomena and atomic-level interactions.
- 🔥 Discusses practical applications and examples, such as burning fuel or wood, to demonstrate how energy transformations are central to chemistry and everyday experiences.
Q & A
What are the three main topics discussed in the lesson?
-The three main topics discussed in the lesson are potential energy, kinetic energy, and work.
Why is understanding the nature of energy important in chemistry?
-Understanding the nature of energy is important in chemistry because it helps grasp the concepts of chemical reactions, electron movements, and bond formations and breakages at a deeper level, which is essential for comprehending the material more effectively.
What is potential energy?
-Potential energy is the potential or ability to do work, often associated with an object's position or condition. It is the stored energy that an object has due to its position in a force field, such as the energy stored in a stretched rubber band or the gravitational potential energy of an object lifted to a height.
How is work defined in physics?
-In physics, work is defined as the energy used when a force moves an object through a distance. It is calculated as the product of the force applied and the distance moved in the direction of the force.
What is the relationship between potential energy and work?
-Potential energy and work are related in that potential energy represents the stored energy that has the potential to be converted into work. When an object with potential energy is allowed to move under the influence of a force, the potential energy is transformed into work done by or on the object.
What is kinetic energy?
-Kinetic energy is the energy of motion. It is the energy that an object possesses due to its movement. The kinetic energy of an object is directly proportional to its mass and the square of its velocity.
Why does nature tend to go from a high energy state to a low energy state?
-Nature tends to go from a high energy state to a low energy state because this transition represents a move towards a more stable configuration. Lower energy states are generally more stable, and systems naturally seek stability, which is why chemical reactions often result in the release of energy as they transition to a lower energy state.
How is the concept of potential energy demonstrated with a rubber band?
-The concept of potential energy is demonstrated with a rubber band by stretching it. When the rubber band is stretched, it stores potential energy due to the tension created by the stretch. If released, this potential energy is converted into kinetic energy as the rubber band snaps back to its relaxed state.
What is the unit of measurement for work, potential energy, and kinetic energy?
-The unit of measurement for work, potential energy, and kinetic energy is the joule (J).
How does the electric force compare to the gravitational force in terms of their equations?
-The equations for electric force and gravitational force are similar in form. Both involve a constant (k for electric force and G for gravitational force), the quantities related to the objects (masses for gravity, charges for electricity), and the distance between the objects. The main difference is that electric force can be both attractive and repulsive, depending on the charges involved, while gravity is always attractive.
What is the significance of the conservation of energy principle in the context of this lesson?
-The conservation of energy principle states that energy cannot be created or destroyed, only transformed from one form to another. In the context of this lesson, it means that potential energy can be converted into kinetic energy or work, and vice versa, without a loss or gain in the total amount of energy in a closed system.
Outlines
🌟 Introduction to the Nature of Energy
The video begins with an introduction to the nature of energy, focusing on the importance of understanding energy concepts in both physics and chemistry. The instructor expresses excitement about teaching this lesson, emphasizing its relevance to the foundational understanding of physical and chemical processes. The three main topics discussed are potential energy, kinetic energy, and work, which are all closely related and share the same units of energy. The lesson aims to clarify these concepts and their interplay, using everyday examples to illustrate the principles and their significance in understanding chemical reactions and energy transformations.
🔋 Potential Energy and Its Relevance
The second paragraph delves deeper into the concept of potential energy, explaining it as the potential to do work. The instructor uses the analogy of a stretched rubber band to demonstrate how potential energy is stored when a force is applied but no movement occurs. The higher the potential energy, the more work can be done when the system is allowed to move. The lesson highlights the importance of potential energy in chemistry, particularly in understanding the behavior of electrons in atoms and the energy changes during chemical reactions. The instructor also emphasizes that potential energy is central to understanding why chemical reactions occur, as nature tends to move towards lower energy states.
💡 Work and Its Relation to Potential Energy
This paragraph introduces the concept of work as energy used when a force moves an object through a distance. The instructor provides a clear definition and illustrates it with the example of pushing a box. Work is calculated as the product of force and distance (in the direction of the force), and its unit is the joule, which is also the unit for potential and kinetic energy. The lesson explains that work is closely related to potential energy, as the potential energy stored in a system can be converted into work when the system moves. The concept is further clarified with the example of a box at different heights, emphasizing that the higher the box, the more work is done against gravity when moving it to a higher position.
🌐 Gravity and Potential Energy
The fourth paragraph transitions the discussion on potential energy to the concept of gravity. The instructor uses the analogy of a person standing on the ground versus on top of a building to explain how height affects potential energy. The higher the height, the greater the potential energy due to gravity. This concept is then related to the behavior of electrons in atoms, where electrons far from the nucleus have higher potential energy and can move towards a lower energy state, akin to falling towards the nucleus. The lesson also introduces the formula for gravitational potential energy, which is the product of mass, gravitational acceleration, and height above the ground.
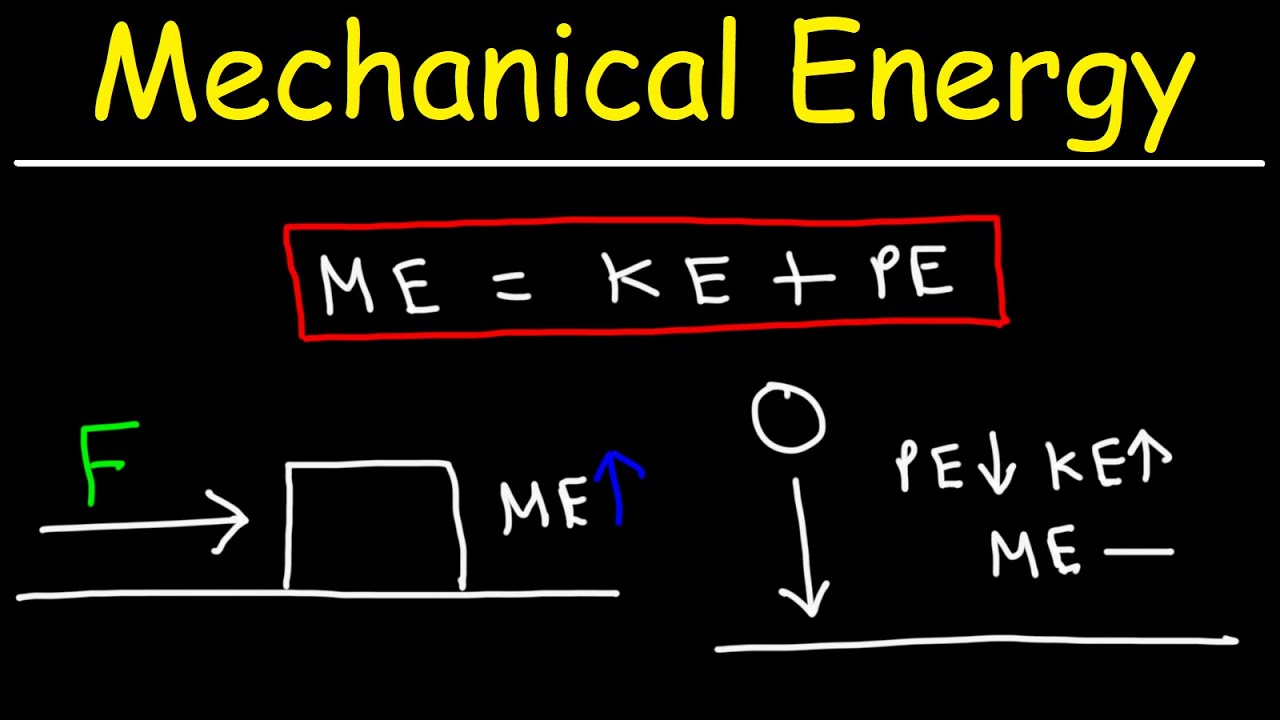
🔄 Energy Transformation and Chemical Reactions
The fifth paragraph continues the discussion on energy states, focusing on how systems naturally tend to move from higher to lower energy states. The instructor uses the example of a roller coaster to illustrate this principle, explaining how potential energy is converted into kinetic energy as the roller coaster descends. The lesson then connects this to chemical reactions, where bonds in molecules represent potential energy that can be converted into kinetic energy or work during a reaction. The transformation of energy is highlighted, with the example of burning wood demonstrating how chemical bonds (potential energy) are broken and released as heat and light (kinetic energy).
🚀 Kinetic Energy and Motion
The sixth paragraph introduces kinetic energy as the energy of motion. The instructor explains that kinetic energy depends on the mass of an object and its velocity, with the formula being one-half times the mass times the velocity squared. The lesson emphasizes that kinetic energy is the form of energy when potential energy is converted into motion. The concept is illustrated with the example of a rubber band being released, where the potential energy stored in the rubber band is transformed into the kinetic energy of the moving object. The lesson concludes with the reiteration of the conservation of energy principle, stating that energy is not created or destroyed but changes from one form to another.
🌈 Conservation of Energy and Its Application
The seventh paragraph discusses the law of conservation of energy, using the example of a roller coaster to demonstrate how potential energy is converted into kinetic energy and then back into potential energy as the ride progresses. The instructor explains that at the top of the roller coaster, the potential energy is at its maximum, while at the bottom, the kinetic energy is at its peak. The lesson highlights the continuous transformation of energy from one form to another, with the total amount of energy remaining constant throughout the process. The concept is then related to chemical reactions, where the potential energy stored in chemical bonds is released as kinetic energy during the reaction.
🔌 Analogy of Gravity and Electric Force
The eighth paragraph draws an analogy between gravity and electric force, showing that despite the electric force being much stronger than gravity, their equations have a similar form. The instructor explains that the force between two objects due to gravity depends on their masses and the distance between them, while the electric force depends on the charges of the objects and the distance. The lesson emphasizes the importance of understanding these forces, as they govern the behavior of particles in chemistry and are key to understanding chemical reactions and the formation of chemical bonds.
🌿 Energy States and Chemical Reactions
The ninth paragraph connects the concepts of potential and kinetic energy to chemical reactions, explaining that reactions often involve the rearrangement of atoms into lower energy configurations. The instructor uses the example of burning wood to illustrate how the potential energy stored in the chemical bonds of the wood is released as heat and light during combustion. The lesson highlights that understanding energy transformations is crucial for predicting the outcomes of chemical reactions and for understanding why certain reactions occur spontaneously while others require external energy input.
📚 Summary and Application of Energy Concepts
The final paragraph summarizes the lesson, emphasizing the importance of understanding the concepts of potential energy, kinetic energy, and work in both physics and chemistry. The instructor encourages the viewer to rewatch the lesson multiple times to fully grasp the connections between these energy concepts and their applications in chemical reactions. The lesson concludes with a reminder that the conservation of energy principle is a fundamental concept that will be repeatedly applied throughout the study of chemistry, and that understanding these energy transformations is key to predicting and understanding chemical behavior.
Mindmap
Keywords
💡Energy
💡Potential Energy
💡Kinetic Energy
💡Work
💡Conservation of Energy
💡Electric Force
💡Chemical Reactions
💡Rubber Band Analogy
💡Gravitational Force
💡Electron Energy Levels
Highlights
The lesson focuses on the nature of energy, emphasizing the importance of understanding energy concepts in both physics and chemistry.
The three main topics discussed are potential energy, kinetic energy, and work, all of which share the same units of energy and are closely related.
Potential energy is introduced as the energy an object possesses due to its position or condition, such as a stretched rubber band or an object raised to a height.
Kinetic energy is the energy of motion, and it depends on the mass of an object and its velocity.
Work is defined as energy used when a force moves an object through a distance, and it is measured in joules.
The concept of potential energy is crucial in chemistry, as it relates to the energy levels of electrons and their movements around the atom.
Chemical reactions often involve the conversion of potential energy into kinetic energy and work, as electrons move and bonds form or break.
Nature tends to move from high energy states to low energy states, which is a fundamental principle in understanding chemical reactions and energy transformations.
The lesson uses gravity as an analogy to explain potential energy, comparing the potential energy of an object at different heights.
The electric force, which governs chemistry, is similar in form to the gravitational force, allowing for the application of gravitational concepts to understand chemical processes.
The conversion of potential energy to kinetic energy is illustrated through the example of a roller coaster, demonstrating the conservation of energy.
The lesson emphasizes the importance of understanding energy at a detailed level to truly grasp the underlying processes in chemistry and physics.
The potential energy equation (mass times gravity times height) and the kinetic energy equation (one-half times mass times velocity squared) are key formulas introduced.
The lesson concludes with a practical example of calculating kinetic energy and a discussion on the energy states of gasoline versus exhaust gases.
The analogy between the electric force in chemistry and the gravitational force in physics is used to explain the behavior of electrons and energy levels.
The lesson highlights the connection between the macroscopic world and atomic-level processes, using everyday experiences to understand complex scientific concepts.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: