Atomic Energy Levels (Micro Lesson for AP Physics)
TLDRThis script simplifies atomic structure, focusing on electrons' movement and energy levels. It explains how electrons absorb energy from photons to jump to higher levels and release energy by emitting photons when falling back to lower levels. The concept of absorption and emission spectra as atomic 'fingerprints' is introduced, illustrating how they help identify elements in distant celestial bodies. The script also touches on the negative energy values of bound electrons and the ionization process when electrons gain enough energy to escape the atom.
Takeaways
- 🔬 The nucleus at the center of the atom contains protons and neutrons, which mostly stay stationary.
- ✨ Electrons are the active particles in an atom, moving around and interacting with other atoms.
- 📊 Energy levels in an atom can be represented using an energy level diagram, which simplifies visualization.
- 📉 Electrons can only exist at specific energy levels and cannot be between these levels.
- 🌟 The ground state is the lowest energy level an electron can have in an atom.
- 💡 Electrons can move to higher energy levels by absorbing photons with the right energy.
- 📉 When electrons fall back to lower energy levels, they emit photons with energy equal to the difference between levels.
- ⚡ The absorption spectrum shows the specific energies that electrons can absorb, acting as a fingerprint for atoms.
- 💡 The emission spectrum consists of the specific energies of photons emitted by electrons falling to lower energy levels.
- 🚀 If an electron gains more than zero energy (positive energy), it becomes unbound from the atom, leading to ionization.
Q & A
What is the primary component of an atom's nucleus?
-The nucleus of an atom is primarily composed of protons and neutrons.
Why are protons and neutrons considered 'boring' in the context of the atom?
-Protons and neutrons are considered 'boring' because they mostly remain stationary within the nucleus and do not participate in the dynamic processes that electrons do, such as moving around and binding with other atoms.
What role do electrons play in an atom?
-Electrons are the 'stars of the show' in an atom, as they are responsible for moving around, jumping between energy levels, and binding with other atoms, which are essential for chemical reactions and interactions.
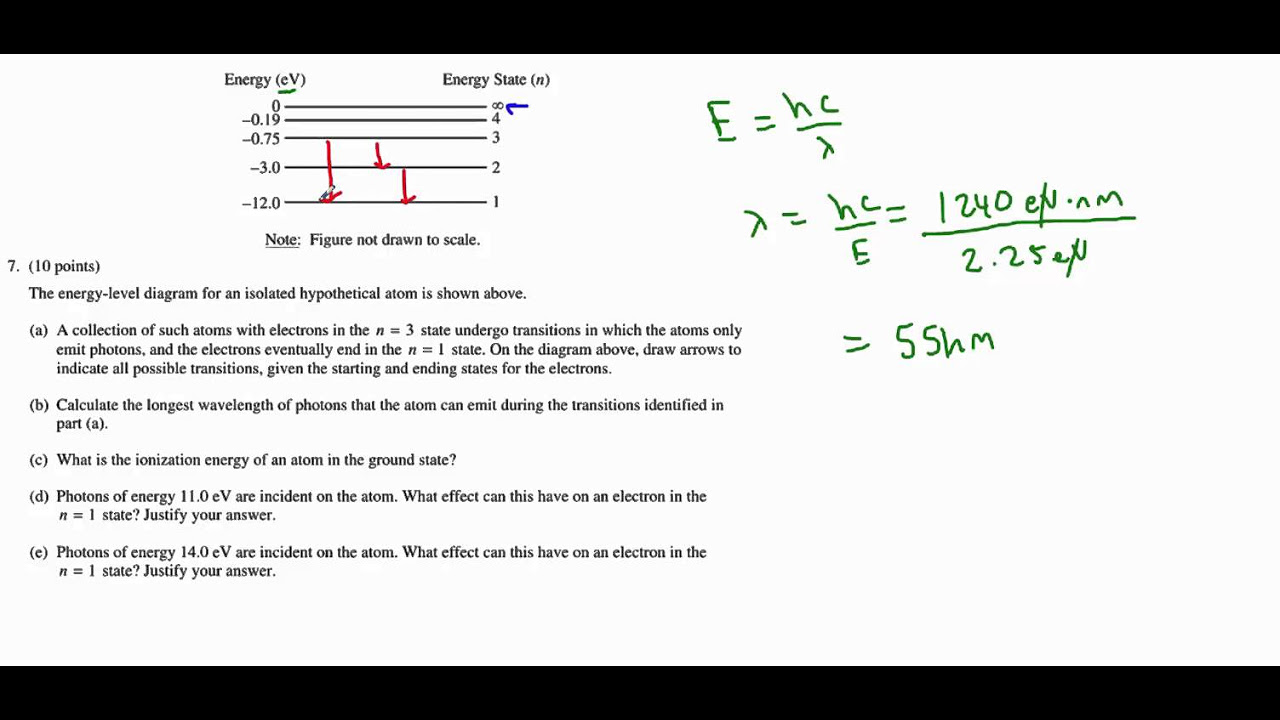
What are the dashed lines in the energy level diagram representing?
-The dashed lines in the energy level diagram represent the different energy levels that an electron can occupy within an atom.
Why is the x-axis in an energy level diagram not meaningful?
-The x-axis in an energy level diagram is not meaningful because moving left or right on the diagram does not represent any physical property; it is simply a visual aid to organize the energy levels.
What is the term for the lowest energy level an electron can have in an atom?
-The lowest energy level an electron can have in an atom is called the ground state.
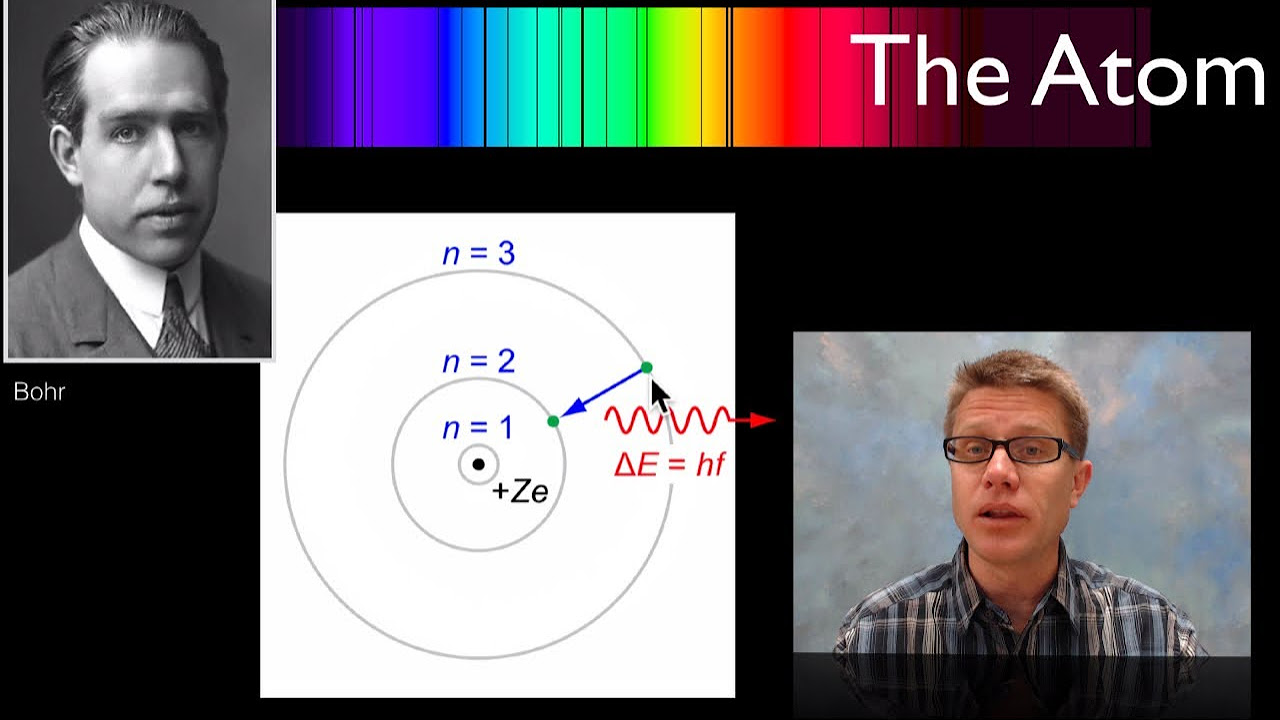
How does an electron move to a higher energy level?
-An electron moves to a higher energy level by absorbing energy from a photon of the right energy. This process is known as excitation.
What happens when an electron at a higher energy level returns to the ground state?
-When an electron at a higher energy level returns to the ground state, it emits a photon with energy equal to the difference between the two energy levels.
Why can't an electron exist between energy levels in an atom?
-An electron cannot exist between energy levels because energy levels are quantized, meaning electrons can only occupy specific, discrete energy levels and not any arbitrary energy in between.
What is the significance of an atom's absorption spectrum?
-An atom's absorption spectrum is significant because it acts as a 'fingerprint' for the atom, allowing scientists to identify the presence of specific elements by observing which wavelengths of light are absorbed.
How are the energies of electrons in real atoms represented?
-In real atoms, the energies of electrons are represented as negative values, indicating that they are bound to the atom. The more negative the energy, the more tightly bound the electron is.
What happens when an electron has more than zero electron volts of energy?
-When an electron has more than zero electron volts of energy, it has positive energy and is no longer bound to the atom. This results in ionization, where the electron is free to leave the atom.
Why are the energy levels of real atoms closer together as they approach zero electron volts?
-The energy levels of real atoms get closer together as they approach zero electron volts because the potential energy of the electron increases as it gets closer to the nucleus, resulting in a denser distribution of energy levels.
What is the relationship between an atom's emission spectrum and its absorption spectrum?
-An atom's emission spectrum and absorption spectrum are related because they both involve the same set of energy levels. The emission spectrum shows the wavelengths or energies of photons emitted when electrons fall from higher to lower energy levels, while the absorption spectrum shows the wavelengths or energies absorbed when electrons move to higher energy levels.
Outlines
🌟 Electron Behavior and Energy Levels
This paragraph explains the basic structure of an atom, highlighting the role of electrons and their energy levels. It introduces the concept of an energy level diagram, which is a simplified way to represent the different energy states an electron can occupy within an atom. The paragraph clarifies that electrons can only exist at specific energy levels and describes the process of electrons absorbing energy from photons to jump to higher levels, as well as releasing energy by emitting photons when they fall back to lower levels. The explanation includes the ground state, excited states, and the discrete nature of energy level transitions. It also touches on the fact that electrons cannot exist between energy levels and that the energy of emitted or absorbed photons is equal to the difference between the energy levels.
🌌 Atomic Spectra and Astrophysical Applications
The second paragraph delves into the implications of atomic energy levels for light absorption and emission, leading to the concept of atomic spectra. It describes how atoms can absorb specific wavelengths of light, leaving dark lines in the spectrum, which serve as a unique fingerprint for each atom. This phenomenon is crucial for astronomers to identify the composition of distant celestial bodies by analyzing the light that passes through them. The paragraph also discusses the emission spectrum, which consists of the wavelengths emitted when electrons fall back to lower energy levels. It points out that the energies involved in both absorption and emission spectra are the same, and the process can be observed in neon lights or gas discharge tubes. The summary also corrects some misconceptions about electron energy levels being positive, explaining that they are actually negative and bound to the atom, and that electrons with more than zero energy are free to leave the atom, a process known as ionization.
Mindmap
Keywords
💡Atom
💡Nucleus
💡Electron
💡Energy Levels
💡Photon
💡Ground State
💡Excited State
💡Absorption Spectrum
💡Emission Spectrum
💡Ionization
Highlights
A simplified model of an atom is presented with the nucleus at the center containing protons and neutrons.
Electrons are the dynamic part of the atom, moving and interacting with other atoms, unlike the stationary nucleus.
Energy levels represented by dashed lines indicate different states an electron can occupy within an atom.
Energy level diagrams are introduced as a method to visualize electron energy without detailed atomic illustrations.
The lack of an x-axis in energy level diagrams is noted, emphasizing that only energy levels matter, not their horizontal position.
Electrons can only exist at specific energy levels and cannot occupy states between these levels.
The ground state is identified as the lowest energy level an electron can have in an atom.
Electrons absorb energy from photons to jump to higher energy levels, exemplified by needing 4 eV to reach the next level.
Electrons naturally fall back to lower energy levels, emitting photons in the process.
The energy of emitted photons corresponds to the difference between energy levels an electron transitions through.
Electrons can jump multiple energy levels at once, emitting photons for each transition.
If an electron absorbs a photon with energy that doesn't match an allowed energy level, the photon passes through unabsorbed.
The absorption spectrum of an atom is described as its 'fingerprint', identifying which wavelengths are absorbed.
Astronomers use absorption spectra to determine the composition of distant celestial objects by analyzing light that passes through them.
Emission spectra are explained as the result of electrons falling to lower energy levels, emitting specific wavelengths of light.
The explanation of how neon lights and gas discharge tubes operate by exciting electrons and observing their emission spectra.
A clarification on the negative values of electron energy levels in atoms, contrasting with the simplified model's positive eV values.
The concept that electrons with more than zero energy are no longer bound to the atom and can be ionized.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: