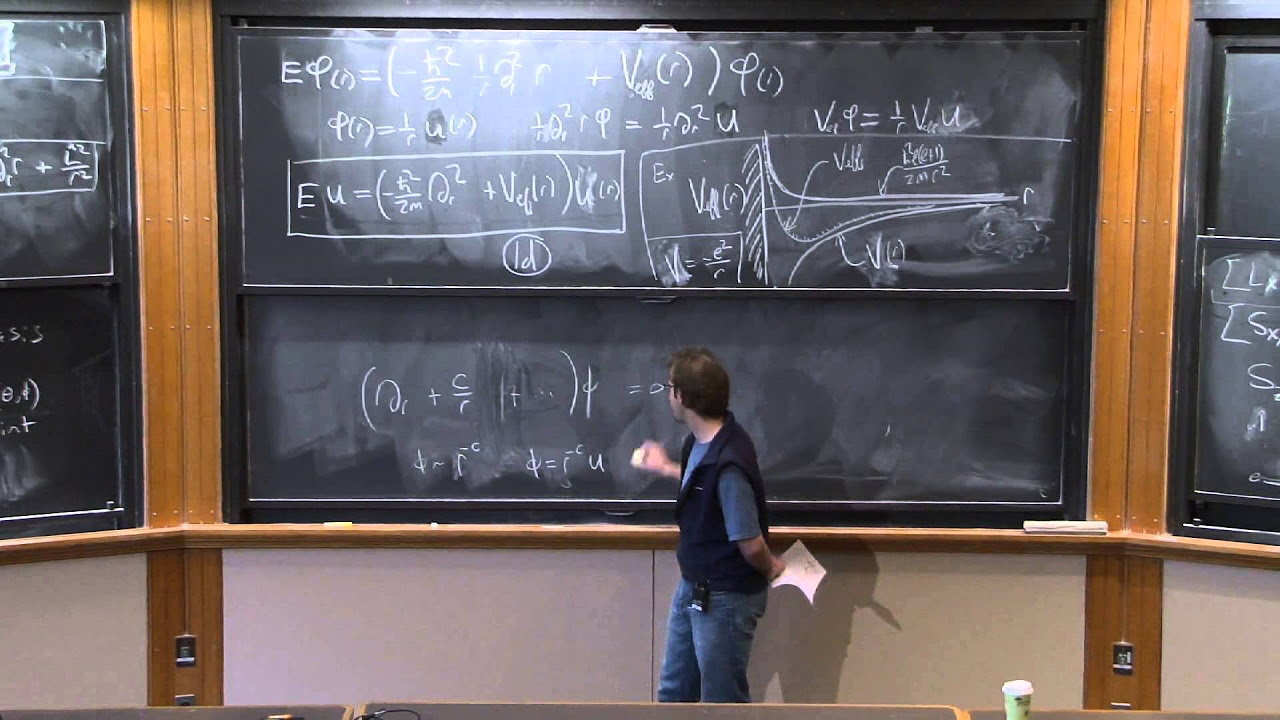9. Atoms V and Atoms in External Fields I
TLDRThis lecture delves into the intricacies of atomic structure, focusing on the effects of external magnetic fields on atoms. It discusses isotope shifts, the significance of mass and volume effects in light and heavier atoms like lithium and rubidium, and the impact of angular momentum on the observability of nuclear deformation. The professor also explores the historical development of hydrogen spectroscopy, the discovery of the Lamb shift, and its implications for quantum electrodynamics (QED). The lecture concludes with an exploration of hyperfine structure in the presence of magnetic fields, providing insights into the complex interplay of atomic and nuclear angular momenta.
Takeaways
- 📚 The lecture discusses the effects on atoms when placed in external magnetic fields, building upon the previous discussion on isotope shifts.
- 🌌 It highlights the importance of understanding isotope shifts in light atoms such as lithium and rubidium, and how they can provide insights into atomic nucleus properties.
- 🔍 The precision of experiments allows for the calculation of mass effects from atomic masses, giving information about the atomic nucleus size even with isotope shifts.
- 🧲 The lecture differentiates between the mass effect and volume effect in isotope shifts, with the mass effect being significantly larger.
- 📉 The comparison between rubidium and lithium shows that the mass effect is 200 times larger in lithium, emphasizing the differences in isotopes' behavior.
- 🤔 It poses a question about the minimum angular momentum required to observe a deformation in a nucleus or any anisotropic shape, discussing the lab frame versus the body-fixed frame.
- 🔄 The discussion on angular momentum and deformation leads to a deeper understanding of the conditions under which an object's shape can be determined or measured.
- 🚀 The lecture provides a historical overview of the development of hydrogen spectroscopy, emphasizing the discovery of the Lamb shift and its implications for QED.
- 🔬 It points out that fundamental limitations in measurements can change over time due to technological advancements and new insights.
- 🌟 The significance of the Lamb shift in hydrogen's energy levels is underscored, showing how technological improvements have allowed for increasingly precise measurements.
- 🔍 The script also touches on the proton radius puzzle, which emerged from a discrepancy between measurements of the proton size from hydrogen spectroscopy and scattering measurements.
Q & A
What is the significance of the isotope shift in the context of the lecture?
-The isotope shift is significant as it allows for the calculation of the mass effect from atomic masses and provides information about the size of the atomic nucleus. The lecture discusses how the isotope shift varies between light atoms like lithium and heavier atoms like rubidium, and how this shift can be used to understand nuclear volume effects.
What is the difference between the mass effect and volume effect in isotope shifts?
-The mass effect in isotope shifts is due to the difference in mass between isotopes, which affects the energy levels of an atom. In contrast, the volume effect is related to the change in electron distribution within the atom due to the change in nuclear size. The lecture mentions that the mass effect is generally larger than the volume effect.
How does the professor use the example of a stick to explain angular momentum and deformation?
-The professor uses the example of a stick to illustrate that if a system can have angular momentum without changing its internal structure, such as spinning the stick, then even at zero angular momentum, it can be inferred to have a deformation that can be measured when angular momentum is added.
What is the body-fixed frame argument regarding deformation of an object?
-The body-fixed frame argument posits that deformation of an object exists inherently, but it may not be measurable at low angular momentum. It suggests that the deformation is always present but only manifests in the lab frame when angular momentum is added.
What is the historical significance of the Lamb shift in the development of QED?
-The Lamb shift was a significant discovery because it demonstrated that the simple model of a hydrogen atom based on the Coulomb law was incomplete. This led to the development of Quantum Electrodynamics (QED), which accounts for the interaction between the electron and the vacuum fluctuations of the electromagnetic field.
How did the advent of lasers impact the measurement of the Lamb shift?
-The advent of lasers allowed for much more precise measurements of the Lamb shift through optical spectroscopy. This new precision enabled scientists to see the Lamb shift directly in the form of split spectral lines, which was previously not possible with radio frequency transitions.
What is the proton radius puzzle mentioned in the lecture?
-The proton radius puzzle refers to the discrepancy between the measurements of the proton's size obtained from hydrogen spectroscopy and those obtained from scattering experiments. The puzzle remains unresolved and challenges our understanding of fundamental physics.
How does the Zeeman effect relate to the addition of an external magnetic field to an atom?
-The Zeeman effect describes how the energy levels of an atom split in the presence of an external magnetic field. This is due to the interaction between the magnetic moment of the atom and the magnetic field, leading to the quantization of the magnetic moment along the field direction.
What is the lambda g-factor, and why is it important in the context of atoms in external magnetic fields?
-The lambda g-factor is a quantity that accounts for the different weights of the magnetic moment due to spin and orbital angular momentum when an atom is in an external magnetic field. It's important because it determines the Zeeman splitting of energy levels in such fields.
What are the two limiting cases for the Zeeman effect in atoms, and how do they differ?
-The two limiting cases for the Zeeman effect are the weak field limit and the strong field limit. In the weak field limit, the Zeeman energy is much smaller than the fine or hyperfine splittings, and the atom's magnetic moment precesses around the total angular momentum. In the strong field limit, the Zeeman energy dominates, and the atom's angular momenta align with the magnetic field direction.
How does the hyperfine structure differ between weak and strong magnetic fields?
-In weak magnetic fields, the hyperfine structure is determined by the coupling of the electron's angular momentum (J) and the nuclear spin (I) to form a total angular momentum (F), which then precesses around the magnetic field. In strong magnetic fields, the hyperfine structure is simplified because the electronic Zeeman energy is much larger than the hyperfine coupling, leading to a direct alignment of J and I with the magnetic field, and the good quantum numbers become mJ and mI.
Outlines
🔬 Atoms and External Magnetic Fields
The professor begins the lecture by discussing the importance of MIT OpenCourseWare and transitions into the topic of atoms, particularly the effects of external magnetic fields on them. The lecture wraps up the discussion on atoms without an external field and introduces the concept of isotope shifts, providing specific examples with lithium and rubidium atoms. The isotope shift is explained in terms of mass and volume effects, and the precision of modern experiments allows for the calculation of these effects and the extraction of information about atomic nuclei sizes.
🌀 Angular Momentum and Deformation of Nucleus
The lecture continues by addressing a question from the previous class regarding the minimum angular momentum required to observe a deformation of a nucleus or any anisotropic shape. The professor explains the concepts of lab frame versus body-fixed frame and how different angular momenta (I=0, 1/2, 1) can reveal information about the object's shape, such as dipole and quadrupole moments. The discussion also explores the philosophical implications of measurement and existence regarding deformation in the lab frame versus the body-fixed frame.
🔍 Spectroscopic Observations and Deformation
This section delves into the nuances of spectroscopic observations and the implications for understanding the deformation of objects like molecules or nuclei. The professor discusses the conditions under which angular momentum can be added without altering the internal structure and how this affects the interpretation of deformation. Examples are provided to illustrate the points, including the case of a weakly bound molecule that cannot withstand angular momentum without disintegrating, as opposed to a stable molecule or a nucleus with excited states that can exhibit deformation.
📚 Historical Development of Hydrogen Spectroscopy
The professor provides a historical overview of the development of hydrogen spectroscopy, highlighting key discoveries such as the Lamb shift and the associated QED corrections. The discussion includes the evolution of technology and experimental techniques that have allowed for increasingly precise measurements, as well as the theoretical advancements that have kept pace with these improvements. The lecture touches on the historical papers that led to the understanding of the need for QED corrections and the technological milestones that enabled their measurement.
🔬 Advances in Hydrogen Spectroscopy and the Proton Radius Puzzle
The lecture continues with an exploration of the advances in hydrogen spectroscopy, focusing on the increasing precision of measurements and the implications for fundamental physics. The discovery of the Lamb shift and its subsequent measurements with higher accuracy are discussed, leading to the introduction of the proton radius puzzle. This puzzle arose from a discrepancy between the proton size determined from hydrogen spectroscopy and that from scattering measurements, which remains an unresolved issue in physics.
🧲 Atoms in External Magnetic Fields: Fine Structure and Lambda g-factor
The professor shifts the focus to the behavior of atoms in external magnetic fields, specifically discussing the fine structure and the lambda g-factor. The lecture explains how the introduction of an external magnetic field adds a new vector to the atomic system, leading to the Zeeman effect. The discussion covers the different g-factors for spin and orbital angular momentum and how they contribute to the magnetic moment of the atom in the presence of a magnetic field.
🌐 Vector Model and Zeeman Hamiltonian
This section delves into the vector model and the Zeeman Hamiltonian to understand the behavior of atoms in weak magnetic fields. The professor explains how the projections of L and S on the J-axis are essential for calculating the Zeeman energies and derives the lambda g-factor using both the vector model and matrix elements. The lecture also touches on the internal magnetic field within an atom due to fine structure and the assumption of the weak field limit for the discussion.
🔗 Hyperfine Structure in Magnetic Fields
The lecture continues with the topic of hyperfine structure in the presence of magnetic fields. The professor introduces the additional vector of nuclear angular momentum and discusses how it interacts with the electronic angular momentum and the external magnetic field. The weak field and strong field limits are explored, along with the derivation of the g-factor for hyperfine structure and the implications for the energy levels of the atom.
📉 High Field Limit and Avoided Crossings
The professor concludes the lecture by examining the high field limit for atoms with hyperfine structure. The discussion focuses on how the electronic Zeeman energy dominates over the hyperfine coupling, leading to a different quantization of angular momentum along the magnetic field. The lecture explains the avoided crossings that occur during the transition from weak to strong field limits and how these can be understood through the diagonalization of the Hamiltonian. The sodium and rubidium 87 atoms serve as examples to illustrate the concepts.
Mindmap
Keywords
💡Isotope Shifts
💡Magnetic Fields
💡Zeeman Effect
💡Hyperfine Structure
💡Lamb Shift
💡Quantum Electrodynamics (QED)
💡Nuclear Volume Effect
💡Angular Momentum
💡Fine Structure
💡g-factor
💡Body-fixed Frame
Highlights
MIT OpenCourseWare's mission to provide high-quality educational resources for free and the option to donate or access additional materials.
Discussion on atoms without an external field and the effects of introducing external magnetic fields.
Explanation of isotope shifts, particularly focusing on lithium and rubidium atoms, and the difference between mass and volume effects.
The precision of experiments allows for the calculation of mass effects from atomic masses, providing information about atomic nucleus size.
Comparison of mass effects in rubidium versus lithium, highlighting the significance of mass change percentage.
Discussion on the minimum angular momentum required to observe deformation in a nucleus or any isotropic shape of an object.
The difference between lab frame and body-fixed frame when observing permanent dipole moments of molecules.
The concept that deformation exists regardless of angular momentum, but may only be measurable at higher momentum.
Historical summary of the development of hydrogen spectroscopy and the discovery of QED through the Lamb shift.
The discovery of the Lamb shift and its precursors, indicating that the understanding of hydrogen structure was evolving even before the Lamb shift was officially discovered.
Fundamental limitations in spectroscopy and how they can disappear over time with advancements in technology.
The evolution of measuring Lamb shifts from radio frequency transitions to optical transitions with increased precision.
The impact of optical metrology and frequency combs on the precision of Lamb shift measurements.
The use of ever-increasing precision in metrology to test the constancy of fundamental constants over time.
The proton radius puzzle arising from a discrepancy between measurements from hydrogen spectroscopy and scattering measurements.
Introduction to atoms in external magnetic fields, discussing the fine structure and the lambda g-factor.
The addition of an external magnetic field as a new vector in the analysis of atomic structure and its impact on the precession of angular momenta.
The Zeeman effect and the introduction of the lambda g-factor in the context of fine and hyperfine structure in atoms.
The derivation of the lambda g-factor using the vector model and its significance in understanding the magnetic moment of the atom.
Transition from weak to strong magnetic fields in the context of hyperfine structure and the phenomenon's impact on fine structure.
Discussion on the weak field and strong field limits of hyperfine structure in atoms in external magnetic fields.
The treatment of the hyperfine structure in the presence of an external magnetic field using perturbation theory.
The graphical representation of the energy level shifts in hyperfine structure due to external magnetic fields, illustrating the avoided crossing concept.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: