Le Chatelier's principle: Worked example | Chemical equilibrium | Chemistry | Khan Academy
TLDRThis video explores Le Chatelier's principle by examining how equilibrium reactions respond to various perturbations. It discusses the effects of adding CO2, increasing container volume, introducing inert gases like argon, adding more calcium carbonate, and using catalysts, highlighting that only changes in concentrations or partial pressures of reactants or products can shift equilibrium.
Takeaways
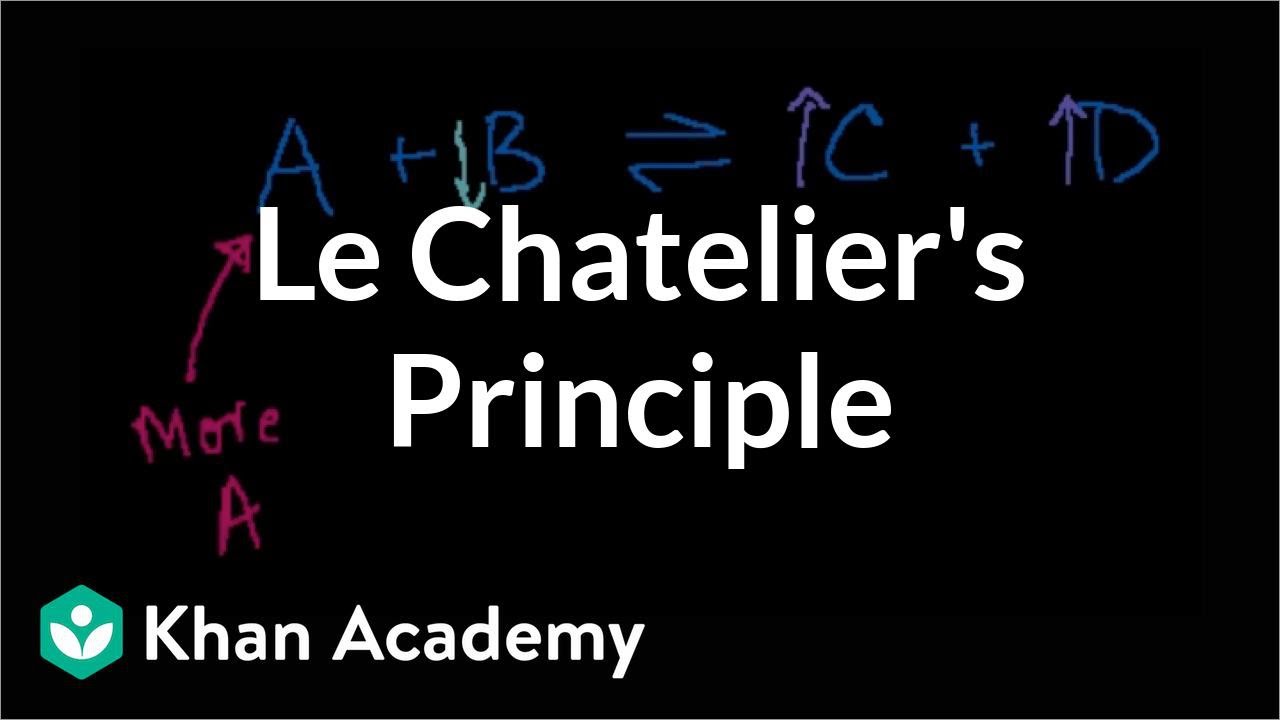
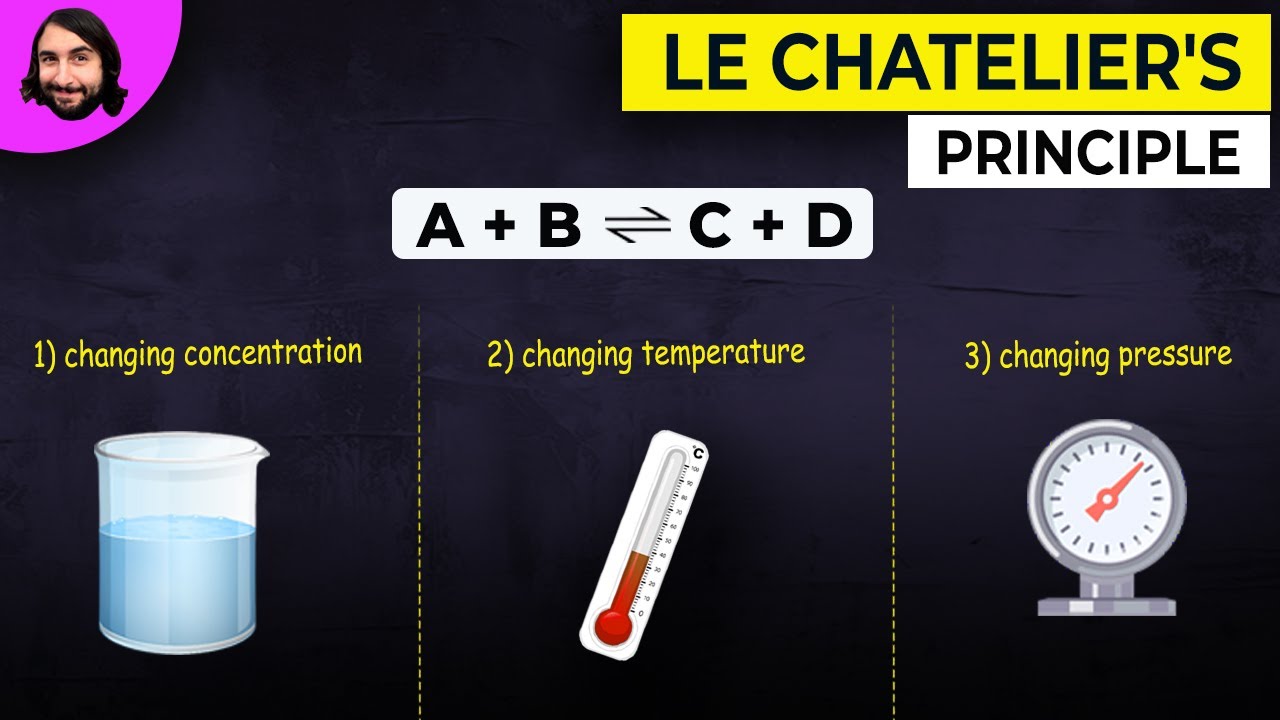
- 🔄 Le Chatelier's principle is used to predict how a reaction at equilibrium will respond to changes in conditions.
- ⚖️ A reaction is at equilibrium when the rates of the forward and reverse reactions are equal, resulting in constant concentrations of reactants and products.
- 🌐 Adding carbon dioxide gas to a reaction at equilibrium will increase its concentration or partial pressure, causing the reaction to shift towards the reverse reaction to counteract the change.
- 📈 The equilibrium constant (Kc) can be expressed in terms of molar concentrations, and (Kp) in terms of partial pressures, both relevant for gases and not including solids.
- 📦 Increasing the volume of a container holding a gas at equilibrium will decrease its partial pressure and concentration, causing the reaction to shift towards the side that produces more gas molecules.
- 💥 Adding inert gas like argon increases the total pressure but does not change the partial pressures of the reacting gases, thus not affecting the equilibrium.
- 💡 Solids, such as calcium carbonate, do not affect the equilibrium of a reaction when added, as their concentration is not included in the equilibrium expressions.
- 🚀 Adding a catalyst to a reaction speeds up both the forward and reverse reactions equally, thus maintaining the equilibrium without shifting the concentrations.
- 🔄 Le Chatelier's principle suggests that a reaction at equilibrium will adjust to counteract any changes in conditions, such as concentration, pressure, or temperature.
- 📚 Understanding the impact of changes in volume, addition of gases, and catalysts on equilibrium is crucial for predicting the behavior of chemical reactions.
Q & A
What is Le Chatelier's principle?
-Le Chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium will shift to counteract the change.
What is meant by a reversible reaction being at equilibrium?
-A reversible reaction is at equilibrium when the rate of the forward reaction (Kf) is equal to the rate of the reverse reaction (Kb), resulting in constant concentrations of reactants and products.
How does adding carbon dioxide gas affect the equilibrium of a reaction?
-Adding carbon dioxide gas increases its concentration or partial pressure, causing the equilibrium to shift in favor of the reverse reaction, thus increasing the concentration of reactants.
What is the equilibrium constant (Kc) and how is it related to the concentration of CO2?
-The equilibrium constant (Kc) is an expression of the product of the concentrations of the products at equilibrium, excluding solids. For a reaction involving CO2, Kc is the concentration of CO2 at equilibrium.
What is the difference between Kc and Kp in terms of equilibrium expressions?
-Kc is the equilibrium constant expressed in terms of molar concentrations, while Kp is the equilibrium constant expressed in terms of partial pressures, applicable for reactions involving gases.
How does increasing the volume of a container affect the partial pressure and concentration of CO2?
-Increasing the volume of a container decreases the partial pressure and concentration of CO2 because volume is in the denominator of the expressions for both partial pressure and molar concentration.
What happens to the equilibrium when the volume of the container is increased?
-When the volume is increased, the partial pressure and concentration of CO2 decrease, causing the equilibrium to shift towards the products to restore the equilibrium concentration and partial pressure of CO2.
Why does adding argon gas not affect the equilibrium of a reaction involving CO2?
-Adding argon gas, an inert gas, increases the total pressure but does not change the partial pressure of CO2 or the volume, thus not disturbing the equilibrium.
What is the effect of adding more calcium carbonate to a reaction at equilibrium?
-Adding more calcium carbonate, a solid, does not change the CO2 concentrations, and therefore, does not perturb the reaction from equilibrium, resulting in no shift in concentrations.
How does a catalyst influence the rates of the forward and backward reactions?
-A catalyst lowers the activation energy for both the forward and backward reactions, speeding up both. However, since it affects both rates equally, it does not shift the equilibrium.
What is the significance of the energy diagram in understanding the effect of a catalyst on a reaction?
-An energy diagram illustrates the activation barriers for both the forward and backward reactions. A catalyst lowers these barriers, increasing the rates of both reactions without affecting the equilibrium position.
Outlines
🔍 Understanding Le Chatelier's Principle
This paragraph introduces the concept of Le Chatelier's principle, focusing on its application to a reversible reaction at equilibrium. The narrator explains that when a reaction is at equilibrium, the rates of the forward and reverse reactions are equal, maintaining constant concentrations of reactants and products. The example given involves adding carbon dioxide to the system, which increases its concentration or partial pressure. According to Le Chatelier's principle, this perturbation causes the reaction to shift towards the reverse reaction, favoring the formation of reactants. The equilibrium constant, expressed in terms of molar concentration (Kc) or partial pressures (Kp), is discussed, emphasizing that solids are not included in these expressions. The paragraph concludes by examining the effects of increasing the volume of the container, which decreases the partial pressure and concentration of CO2, leading the reaction to shift towards the products to restore equilibrium.
🌐 Effects of Adding Solids, Inert Gases, and Catalysts
The second paragraph delves into the effects of adding solids, inert gases, and catalysts to a reaction at equilibrium. Adding more calcium carbonate, a solid, does not disturb the equilibrium since the equilibrium expressions are determined by the concentration of CO2. Similarly, introducing argon gas, an inert gas, increases the total pressure but does not affect the partial pressure of CO2, thus maintaining the equilibrium. The paragraph also discusses the role of catalysts, which speed up both the forward and reverse reactions by lowering the activation energy barriers, but do not shift the reaction from equilibrium. The key takeaway is that inert gases, solids, and catalysts do not alter the equilibrium state of a reaction, as they do not change the partial pressures or concentrations of the reactants and products involved in the equilibrium expressions.
Mindmap
Keywords
💡Le Chatelier's Principle
💡Equilibrium
💡Carbon Dioxide (CO2)
💡Partial Pressure
💡Equilibrium Constant (K)
💡Volume
💡Inert Gas
💡Calcium Carbonate
💡Catalyst
💡Activation Energy
💡Ideal Gas Law
Highlights
Introduction to applying Le Chatelier's principle to a reversible reaction at equilibrium.
Explanation of dynamic equilibrium where forward and reverse reaction rates are equal.
Impact of adding carbon dioxide gas on the equilibrium, favoring the reverse reaction.
Use of equilibrium constant Kc to express the relationship between product and reactant concentrations.
Exclusion of solids in equilibrium expressions, focusing only on gases and solutions.
Conversion of partial pressures to volume terms using the ideal gas law.
Effect of increasing container volume on partial pressure and molar concentration of CO2.
Le Chatelier's principle's prediction of product favoring in response to decreased CO2 concentration.
Analysis of the inert nature of argon gas and its impact on equilibrium due to increased total pressure.
Clarification that adding argon gas does not change the partial pressure of CO2, thus no equilibrium shift.
Discussion on the equilibrium state remaining unchanged with the addition of calcium carbonate.
Introduction of energy diagrams to visualize the effect of catalysts on reaction rates.
Explanation of how catalysts lower activation energy for both forward and reverse reactions.
Insight that adding a catalyst does not shift the reaction from equilibrium due to equal rate acceleration.
Summary of the key takeaways: inert gases, solids, and catalysts do not cause equilibrium shifts.
Emphasis on the importance of understanding the effects of various perturbations on equilibrium reactions.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: