Limiting reactant example problem 1 | Chemistry | Khan Academy
TLDRThe video script details a chemistry lesson on determining the limiting reactant in the production of methanol from carbon monoxide and hydrogen. It guides through verifying the balanced equation, calculating moles of reactants, identifying carbon monoxide as the limiting reactant, and computing the mass of methanol produced and excess hydrogen remaining. The process involves stoichiometric calculations and emphasizes mass conservation, concluding with the production of 406 grams of methanol and 15 grams of unreacted hydrogen.
Takeaways
- 🔍 The script discusses the production of methanol from carbon monoxide and hydrogen, emphasizing it as a fuel in racing cars and fuel cells.
- 📝 The problem presented involves determining the mass of methanol that can be produced and identifying the excess reactant after the limiting reactant is consumed.
- 🧪 The script provides a step-by-step guide on how to approach a limiting reactant problem in chemistry, starting with checking the balance of the chemical equation.
- 📚 It explains the importance of converting grams of reactants to moles using their respective molecular weights to determine the limiting and excess reactants.
- 📉 The molecular weights of carbon monoxide (28 g/mol) and hydrogen (2 g/mol) are calculated to convert the given grams into moles.
- ⚖️ The script calculates 12.7 moles of carbon monoxide and 32.5 moles of hydrogen from the given grams, revealing that hydrogen is in excess.
- 🔄 The stoichiometric ratio from the balanced equation is used to determine that carbon monoxide is the limiting reactant, with a 2:1 ratio to hydrogen.
- 📊 The script performs calculations to show that 406 grams of methanol can be produced from the limiting reactant, carbon monoxide.
- 🔢 The mass of the excess reactant, hydrogen, is determined by mass conservation, showing that 15 grams of hydrogen remain unreacted.
- 🔄 An alternative method is presented to find the mass of the excess reactant by calculating the required moles of hydrogen for the consumed carbon monoxide and subtracting from the initial amount.
- 📝 The script concludes by emphasizing the importance of understanding chemical reactions, stoichiometry, and the concept of limiting and excess reactants in chemistry.
Q & A
What is the purpose of the reaction involving carbon monoxide and hydrogen?
-The reaction involving carbon monoxide and hydrogen is used to produce methanol, which is utilized as a fuel in racing cars and fuel cells.
What are the reactants given in the problem?
-The reactants given in the problem are 356 grams of carbon monoxide and 65 grams of molecular hydrogen.
What is the role of the limiting reactant in a chemical reaction?
-The limiting reactant is the reactant that is present in the smallest amount relative to the stoichiometric ratio required by the balanced chemical equation. It dictates the maximum amount of product that can be formed in the reaction.
How is the balanced chemical equation checked for the given reaction?
-The balanced chemical equation is checked by ensuring that the number of atoms of each element on the reactant side matches the number on the product side.
What is the molecular weight of carbon monoxide?
-The molecular weight of carbon monoxide (CO) is 28 grams per mole, which is calculated by adding the atomic weight of carbon (12) and oxygen (16).
How many moles of carbon monoxide are present in 356 grams?
-To find the number of moles of carbon monoxide in 356 grams, divide the mass (356 grams) by the molar mass (28 grams/mole), resulting in approximately 12.71 moles.
What is the molecular weight of molecular hydrogen?
-The molecular weight of molecular hydrogen (H2) is 2 grams per mole, as each hydrogen atom has an atomic weight of 1 and there are two atoms in a molecule.
How many moles of hydrogen are present in 65 grams?
-To find the number of moles of hydrogen in 65 grams, divide the mass (65 grams) by the molar mass (2 grams/mole), resulting in 32.5 moles.
What is the stoichiometric ratio of hydrogen to carbon monoxide in the reaction?
-The stoichiometric ratio of hydrogen to carbon monoxide in the reaction is 2:1, meaning two moles of hydrogen are required for every mole of carbon monoxide.
Which reactant is the excess reactant in this reaction?
-Hydrogen is the excess reactant in this reaction because there are more moles of hydrogen (32.5 moles) than the stoichiometric ratio requires (2 moles of hydrogen per mole of carbon monoxide).
How much methanol can be produced from the given reactants?
-Since carbon monoxide is the limiting reactant with 12.7 moles, and the stoichiometric ratio indicates that 1 mole of carbon monoxide produces 1 mole of methanol, 12.7 moles of methanol can be produced.
What is the mass of methanol produced from 12.7 moles?
-The mass of methanol produced can be calculated by multiplying the number of moles (12.7 moles) by the molar mass of methanol (32 grams/mole), resulting in approximately 406 grams.
What is the mass of the excess reactant remaining after the reaction?
-The mass of the excess reactant (hydrogen) remaining can be calculated by subtracting the mass of methanol produced (406 grams) from the initial total mass of reactants (421 grams), resulting in approximately 15 grams of hydrogen remaining.
Outlines
🔍 Identifying the Limiting Reactant in Methanol Production
This paragraph introduces the chemical reaction for producing methanol, a fuel used in racing cars and fuel cells, from carbon monoxide and hydrogen. The focus is on determining the limiting reactant and the mass of methanol that can be produced. The process involves checking the balanced chemical equation and converting the given masses of reactants (356 grams of carbon monoxide and 65 grams of hydrogen) into moles. The molecular weights of carbon monoxide (28 g/mol) and hydrogen (2 g/mol) are used to calculate the moles of each reactant. The stoichiometric ratio from the balanced equation (1 mole of carbon monoxide reacts with 2 moles of hydrogen) is then used to identify the limiting reactant, which dictates the amount of product formed.
🧪 Calculating Moles and Determining the Excess Reactant
The second paragraph delves into the calculation of moles for both carbon monoxide and hydrogen, leading to the identification of the excess reactant. With 356 grams of carbon monoxide, 12.7 moles are determined, and with 65 grams of hydrogen, 32.5 moles are calculated. The stoichiometric ratio from the balanced equation is then used to compare these moles, revealing that hydrogen is in excess (2.56 moles of hydrogen for every mole of carbon monoxide). Consequently, carbon monoxide is identified as the limiting reactant, as there is not enough to react with all the hydrogen provided. This understanding is crucial for determining the maximum amount of methanol that can be synthesized.
📚 Using Stoichiometry to Calculate Methanol Production
This paragraph explains how the stoichiometric ratio from the balanced chemical equation is used to calculate the amount of methanol produced. Since carbon monoxide is the limiting reactant, the 12.7 moles of carbon monoxide available will directly determine the production of 12.7 moles of methanol. The molecular weight of methanol (32 g/mol) is then used to convert these moles into grams, resulting in 406.4 grams of methanol. This step is crucial for understanding the maximum yield of methanol from the given reactants.
🔄 Mass Conservation and Calculation of Remaining Reactant
The final paragraph focuses on the conservation of mass and the calculation of the remaining reactant after the reaction. Starting with 421 grams of reactants (356 grams of carbon monoxide and 65 grams of hydrogen), the production of 406 grams of methanol implies that 15 grams of reactant remain unconsumed. Since hydrogen is the excess reactant, this remaining mass is attributed to hydrogen. An alternative method of calculating the remaining hydrogen by using the stoichiometric ratio and the moles of carbon monoxide is also presented, leading to a slightly different result due to rounding differences. This discussion reinforces the concept of mass conservation in chemical reactions.
Mindmap
Keywords
💡Methanol
💡Carbon Monoxide
💡Hydrogen
💡Limiting Reactant
💡Excess Reactant
💡Stoichiometric Ratio
💡Molecular Weight
💡Moles
💡Balanced Equation
💡Mass Conservation
Highlights
Methanol can be produced by the reaction of carbon monoxide and hydrogen, which is used as fuel in racing cars and fuel cells.
The problem involves determining the limiting reactant and calculating the mass of methanol produced and excess reactant remaining.
The balanced chemical equation is confirmed to have 1 carbon, 1 oxygen, and 4 hydrogens on both sides.
The molecular weights of carbon monoxide (28) and hydrogen (2) are calculated to convert grams to moles.
356 grams of carbon monoxide is converted to 12.7 moles, and 65 grams of hydrogen is converted to 32.5 moles.
The stoichiometric ratio of the reactants is analyzed, with 2.56 moles of hydrogen available for every mole of carbon monoxide.
Carbon monoxide is identified as the limiting reactant, with hydrogen in excess due to the higher molar ratio.
The methanol production is limited by the 12.7 moles of carbon monoxide available.
12.7 moles of carbon monoxide will produce 12.7 moles of methanol based on the balanced equation.
The atomic weight of methanol (32) is used to calculate the mass of methanol produced from the moles.
406 grams of methanol is calculated to be produced from the limiting reactant.
Mass conservation is applied to determine the mass of excess reactant remaining after the reaction.
15 grams of hydrogen is determined to be the excess reactant remaining after the reaction.
An alternative method of calculating the excess reactant by using the stoichiometric ratio and moles of reactants is explained.
The alternative method confirms 14.2 grams of hydrogen remaining, consistent with the mass conservation approach.
The problem-solving process emphasizes the importance of identifying the limiting reactant and applying stoichiometry to chemical reactions.
Transcripts
Browse More Related Video

Limiting Reactant Practice Problem (Advanced)

Step by Step Stoichiometry Practice Problems | How to Pass Chemistry

Stoichiometry: Limiting Reactant, Left Over Excess Reactant, Percent Yield | Study Chemistry With Us

How to Find Limiting Reactants | How to Pass Chemistry
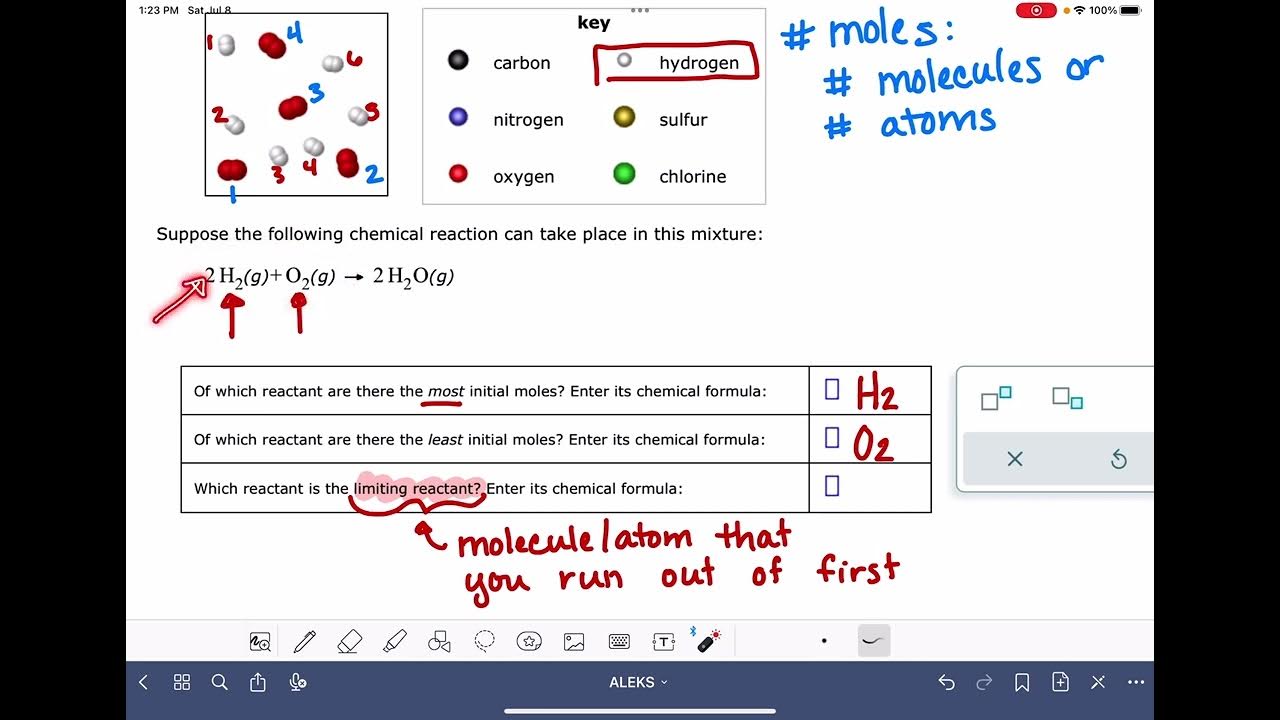
ALEKS: Identifying the limiting reactant in a drawing of a mixture
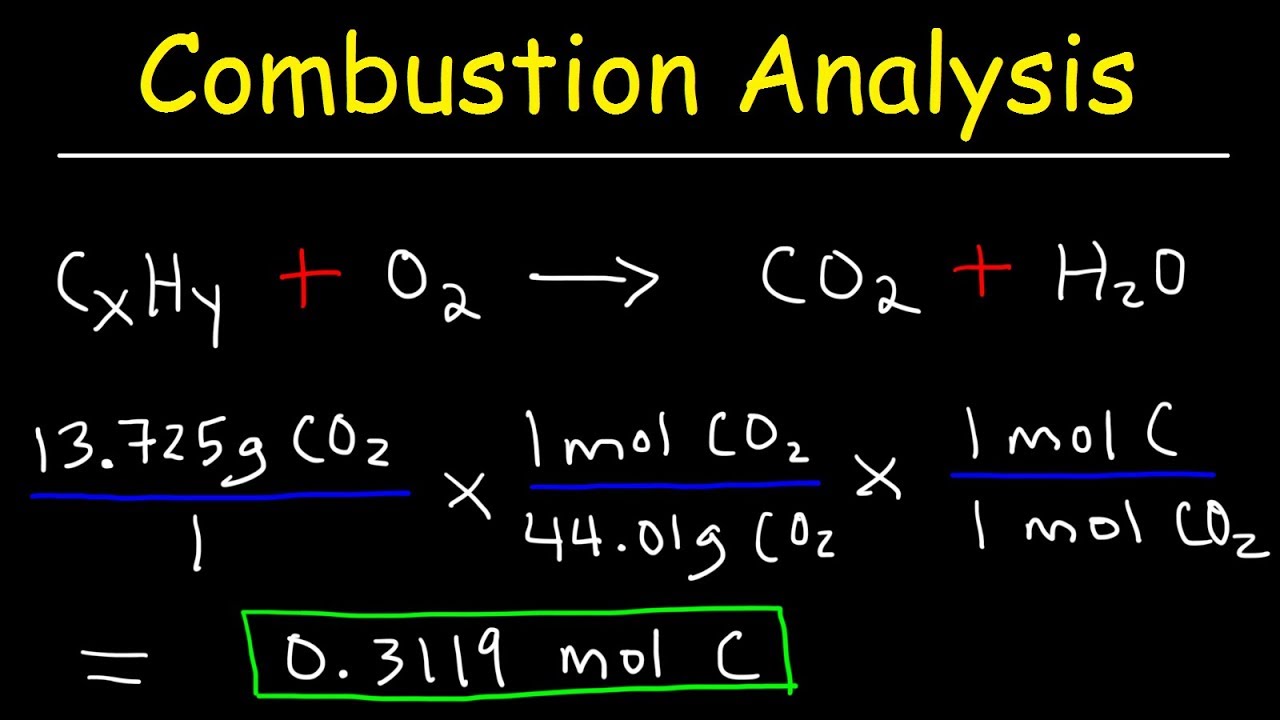
Introduction to Combustion Analysis, Empirical Formula & Molecular Formula Problems
5.0 / 5 (0 votes)
Thanks for rating: