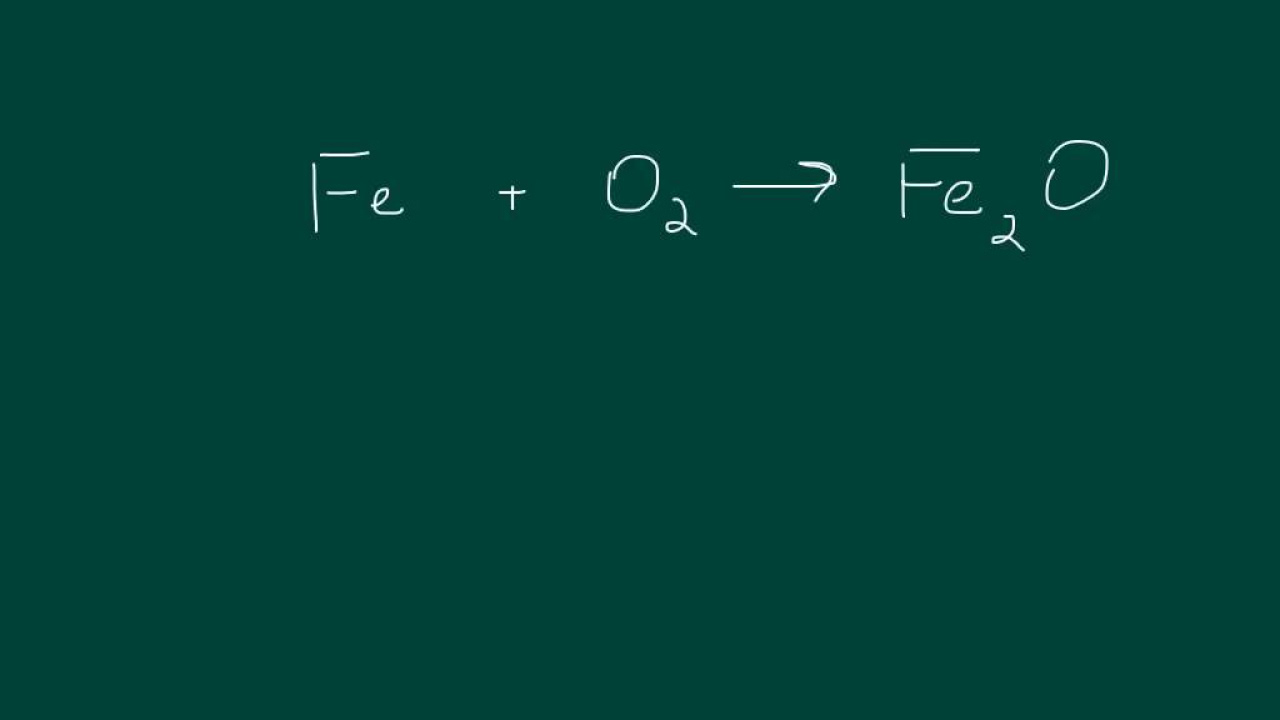GCSE Chemistry Revision "Balancing Chemical Equations"
TLDRIn this video, you'll learn how to balance chemical equations with an effective method. The lesson starts with a review of chemical formulas, explaining the significance of capital letters and small numbers. The instructor demonstrates how to balance equations by ensuring the number of atoms of each element is equal on both sides. Through examples, you'll see how to use large numbers to achieve balance without altering small numbers. You'll also get practice questions to test your understanding. By the end, you'll be confident in balancing chemical equations.
Takeaways
- 🧪 Chemical formulas represent compounds, with capital letters indicating elements and subscript numbers indicating the count of atoms.
- 🔢 The small subscript numbers in a chemical formula must not be altered, as changing them results in a different molecule.
- 📚 A balanced chemical equation has the same number of atoms for each element on both sides of the equation.
- ⚖️ In the provided example, the equation of calcium plus chlorine producing calcium chloride is balanced because it maintains equal atom counts.
- 🚫 You cannot change the small numbers in a chemical formula, but you can use large numbers (coefficients) in front of the formula to balance the equation.
- 🔍 To balance an equation, count the atoms of each element on both sides and adjust the coefficients until the counts are equal.
- 📐 The example of sodium plus iodine producing sodium iodide was used to demonstrate the process of balancing an equation by adjusting coefficients.
- 📝 In exams, you might only be asked to balance a part of a chemical equation, not the entire equation.
- 📑 The script provides examples for practice, such as balancing the reaction between calcium oxide and hydrochloric acid to form calcium chloride and water.
- 🤓 Another example given is the reaction between iron oxide and carbon monoxide to produce iron and carbon dioxide, which requires balancing by adjusting coefficients.
- 📚 The video suggests using a revision workbook for more practice on balancing chemical equations, accessible via a provided link.
Q & A
What is the main topic of the video?
-The main topic of the video is teaching the method to balance chemical equations.
What is a chemical formula and how does it represent elements in a compound?
-A chemical formula represents a compound and shows the elements it contains with capital letters. The subscript numbers indicate the number of atoms of each element in the compound.
Why is it important not to change the small numbers in a chemical formula?
-Changing the small numbers in a chemical formula would produce a different molecule, which is not allowed in chemistry as it alters the identity of the compound.
What does a balanced chemical equation signify?
-A balanced chemical equation signifies that the number of atoms of each element is the same on both sides of the equation, ensuring the conservation of mass.
How can you balance the chemical equation for the reaction of sodium with iodine to form sodium iodide?
-To balance the equation, place a coefficient of 2 in front of sodium iodide to have two iodine atoms on the right side, and then place a coefficient of 2 in front of sodium to have two sodium atoms on the left side, matching the two on the right.
What is the purpose of using large numbers in front of chemical formulas in a reaction?
-Large numbers are used to indicate the number of molecules or moles of a substance involved in a chemical reaction, and they help in balancing the equation.
What is the significance of the rule that you cannot change the small numbers in a chemical formula during the balancing process?
-The rule ensures that the chemical identity of the substances involved in the reaction is maintained. Changing the small numbers would imply altering the fundamental structure of the molecules, which is not permissible.
In the video, what is an example of a balanced chemical equation provided?
-The example given is the reaction of calcium with chlorine to form calcium chloride, which is balanced as there is one atom of calcium and two atoms of chlorine on both sides of the equation.
How can you approach balancing a chemical equation when given only a part of it to balance in an exam?
-Focus on the part of the equation provided and adjust the coefficients in front of the molecules to ensure that the number of atoms of each element is the same on both sides of the equation.
What are the two examples given in the video for the viewers to practice balancing chemical equations?
-The two examples are the reaction of calcium oxide with hydrochloric acid to produce calcium chloride and water, and the reaction of iron oxide with carbon monoxide to produce iron and carbon dioxide.
What resource is mentioned in the video for additional practice on balancing chemical equations?
-The video mentions a revision workbook with more questions on balancing chemical equations, which can be accessed by clicking on the provided link.
Outlines
🧪 Understanding Chemical Formulas and Balancing Equations
This paragraph introduces the concept of chemical formulas and the importance of balancing chemical equations. It explains the meaning of the numbers in chemical formulas, which indicate the quantity of atoms of each element in a compound. The paragraph emphasizes the rule that these numbers cannot be changed as it would result in a different molecule. An example is given with sodium carbonate, where the formula indicates three elements and their respective atom counts. The video script then moves on to demonstrate how to balance a chemical equation, using the reaction between calcium and chlorine to form calcium chloride as an example. It shows that a balanced equation has the same number of atoms for each element on both sides of the equation.
🔍 Balancing Chemical Equations: A Step-by-Step Guide
This paragraph continues the discussion on balancing chemical equations, providing a step-by-step method to achieve balance. It starts with an unbalanced equation involving sodium and iodine to form sodium iodide, and explains how to count and balance the atoms of each element. The paragraph clarifies that while small numbers in a chemical formula cannot be altered, large numbers can be used to adjust the quantity of molecules. The example illustrates how to correct the imbalance by adding coefficients to the reactants and products. The script also mentions that in exams, students are typically given only part of an equation to balance, and provides two practice examples involving calcium oxide and hydrochloric acid, and iron oxide and carbon monoxide. The summaries of these examples show the process of adding coefficients to balance the equations, ensuring an equal number of atoms for each element on both sides.
Mindmap
Keywords
💡Chemical Equations
💡Chemical Formula
💡Stoichiometry
💡Element
💡Reactants
💡Products
💡Balancing
💡Coefficients
💡Molecules
💡Atoms
💡Revision Workbook
Highlights
Introduction to the concept of chemical formula and its importance in balancing chemical equations.
Explanation of how capital letters in a chemical formula indicate the presence of elements.
Clarification of the role of subscript numbers in a chemical formula, representing the number of atoms of each element.
Emphasis on the rule that subscript numbers in a chemical formula must not be altered.
Introduction of the concept of using large numbers to represent multiple molecules of a compound.
Definition and demonstration of a balanced chemical equation with an example of calcium and chlorine.
Guidance on how to count atoms of each element to check for balance in a chemical equation.
Methodology for balancing chemical equations by adjusting large numbers in front of compounds.
Illustration of the process to balance a chemical equation using sodium and iodine as an example.
Explanation of the restrictions on changing subscript numbers versus the flexibility with large numbers.
Advice on common exam tasks related to balancing chemical equations and what to expect.
Presentation of a practice example involving calcium oxide, hydrochloric acid, calcium chloride, and water.
Step-by-step solution to balance the practice example, emphasizing the importance of hydrogen and chlorine balance.
Introduction of a second practice example with iron oxide reacting with carbon monoxide to produce iron and carbon dioxide.
Detailed walkthrough of balancing the iron oxide and carbon monoxide reaction, focusing on carbon and oxygen atoms.
Promotion of the revision workbook for additional practice on balancing chemical equations.
Conclusion summarizing the lesson on balancing chemical equations and encouraging further practice.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: