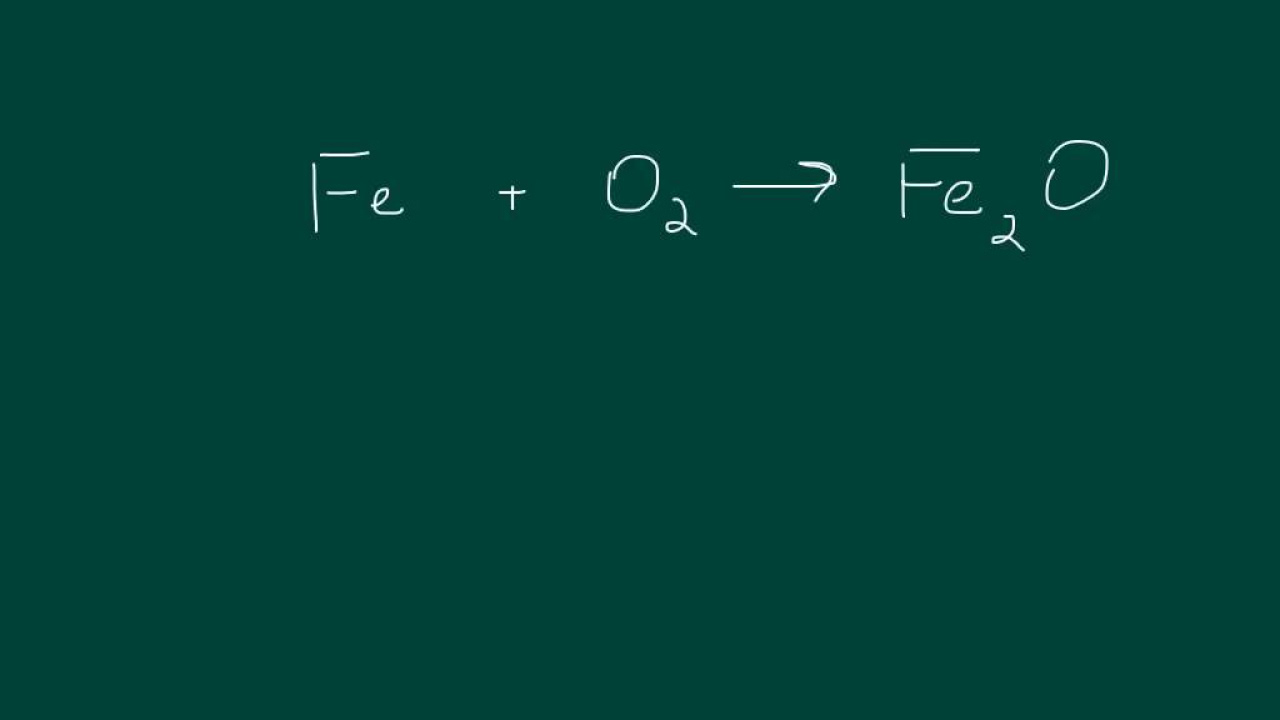Balancing Chemical Equations Practice Problems
TLDRThe video script is an engaging walkthrough of balancing chemical equations, starting with basic examples and gradually increasing in complexity. The presenter emphasizes the importance of tracking the number of atoms for each element on both sides of the equation and demonstrates how to use coefficients to achieve balance. Common misconceptions, such as altering subscripts, are addressed, and the process is illustrated with multiple elements and compounds, including those with parentheses indicating multiplication. The summary highlights the step-by-step approach to balancing equations, which becomes more nuanced with each subsequent problem, ultimately requiring multiple steps and strategic consideration of element balance.
Takeaways
- 🔍 **Balancing Equations**: The process involves adjusting coefficients to ensure equal numbers of each atom on both sides of the equation.
- ✅ **Coefficients vs. Subscripts**: You can only change coefficients (numbers in front of elements or compounds), not subscripts (numbers within the chemical formula).
- 📊 **Starting with Basics**: Begin balancing equations with elements that are present in simpler compounds or those that appear alone.
- 🔁 **Multi-Step Process**: Some equations require more than one step to balance, often starting with elements that are part of multiple compounds.
- 🚫 **No Changing Subscripts**: A common misconception is changing subscripts to balance equations, which is not allowed.
- 🌟 **Oxygen and Hydrogen**: When balancing, focus on elements that are part of every compound in the equation, like oxygen and hydrogen, to simplify the process.
- ➕ **Adding Coefficients**: To balance elements that appear alone, add coefficients in front of the element to match the number on the other side.
- 🔄 **Criss-Cross Method**: For balancing hydrogen and oxygen, the criss-cross method (multiplying by respective coefficients) can be used.
- 📐 **Parentheses**: When an equation has parentheses, it means that the entire content within is multiplied by the number outside the parentheses.
- 🔀 **Element Order**: Often, it's strategic to balance elements in a specific order, such as starting with those that are part of multiple compounds and leaving elements alone that appear only once.
- ⚖️ **Final Check**: After applying coefficients, ensure that the equation is balanced by checking that the number of atoms for each element is equal on both sides.
Q & A
What is the first step in balancing a chemical equation?
-The first step is to make a chart to keep track of the number of atoms of each element on both sides of the equation.
Why can't you change the subscripts in a chemical equation to balance it?
-Subscripts represent the number of atoms of an element within a molecule, and changing them would alter the identity of the compound, which is not allowed in balancing equations.
How do you balance a chemical equation with multiple elements and compounds?
-You balance it by adjusting the coefficients (the numbers in front of the compounds) while ensuring that the number of atoms for each element is the same on both sides of the equation.
What is a common misconception about balancing chemical equations?
-A common misconception is that you can change the subscripts of elements in compounds to balance the equation, which is not permitted.
How do you handle elements that are part of multiple compounds in an equation?
-You may choose to balance those elements last, as adjusting coefficients for other compounds can affect the count of these shared elements.
What does it mean if you have parentheses in a chemical equation?
-Parentheses indicate that the entire compound or element inside them is multiplied by the number following the parentheses.
What is a coefficient in a chemical equation?
-A coefficient is a number placed in front of an element or compound in a chemical equation to balance the number of atoms on both sides.
Why is it important to balance the number of oxygen atoms carefully when they are part of multiple compounds?
-Oxygen is often involved in many reactions, and accurately balancing its atoms ensures the equation represents the correct stoichiometry of the reaction.
null
-null
Can you balance a chemical equation in one step or does it often require multiple steps?
-While simple equations can sometimes be balanced in one step, more complex equations often require multiple steps to ensure all elements are balanced.
What is the criss-cross method mentioned in the script?
-The criss-cross method is a technique used to balance chemical equations by multiplying the coefficients in a way that the product of the two coefficients equals the total number of atoms of the element that needs balancing.
How do you balance an equation that includes elements that are by themselves, like copper in the script?
-You balance these elements last, after balancing the elements that are part of compounds. You adjust the coefficients for these elements to match the total number of atoms on both sides of the equation.
Outlines
🧪 Balancing Chemical Equations with Xenon and Fluorine
The paragraph introduces the concept of balancing chemical equations through practice problems. It begins with a basic example involving xenon and fluorine, emphasizing the importance of tracking atoms on both sides of the equation. A chart is used to compare the number of xenon and fluorine atoms, revealing an imbalance. To correct this, coefficients are added to balance the equation, resulting in six fluorine atoms on both sides. The paragraph also clarifies a common misconception about changing subscripts, stressing that only coefficients can be altered, not subscripts, to achieve balance.
🔍 Advanced Balancing with Multiple Elements and Oxygen
This paragraph tackles more complex chemical equations involving multiple elements such as silver, hydrogen, sulfur, potassium, oxygen, hydrogen, carbon, sodium, chlorine, iron, and parentheses to denote multiples of compounds. The process involves balancing each element step by step, with special attention given to oxygen due to its presence in all compounds. The paragraph also demonstrates how to balance equations that require more than one step, using coefficients to adjust the number of atoms until the equation is balanced on both sides.
🤔 Balancing Equations with Parentheses and Multiple Steps
The final paragraph deals with chemical equations that include parentheses, which indicate that the entire content within is multiplied by the number preceding the parentheses. The paragraph focuses on balancing equations with multiple elements like iron, oxygen, carbon, sodium, and hydrogen. It explains how to address imbalances by adjusting coefficients, starting with elements that are easier to balance and then moving on to more complex scenarios. The process involves strategic multiplication of elements and compounds to achieve balance across the equation, with a focus on understanding how changes in one part of the equation can affect the overall balance.
Mindmap
Keywords
💡Balancing Chemical Equations
💡Coefficients
💡Subscripts
💡Elements and Compounds
💡Conservation of Mass
💡Mole Ratio
💡Oxidation States
💡Isolation Method
💡Criss-Cross Method
💡Practice Problems
💡Law of Conservation of Charge
Highlights
The process of balancing chemical equations is introduced with basic examples.
A chart is used to keep track of atoms of elements on both sides of the equation.
Coefficients are added to balance the number of atoms for each element.
Subscripts cannot be changed; only coefficients can be added to balance equations.
The importance of not changing subscripts in chemical formulas is emphasized.
An example with silver, hydrogen, and sulfur is used to demonstrate balancing.
The concept of balancing elements with multiple atoms in compounds is explained.
An equation with potassium, oxygen, hydrogen, and carbon is balanced step by step.
The strategy of balancing elements in a compound separately is discussed.
An example with sodium and chlorine demonstrates balancing with multiple steps.
The transcript covers balancing equations with more than one element in a compound.
The use of parentheses to indicate multiplication of all elements within is explained.
An example with iron, oxygen, and carbon is used to show balancing with coefficients.
The transcript explains how to handle elements that are part of multiple compounds.
An equation with silicon, oxygen, and carbon is balanced, focusing on elements present in multiple compounds.
The transcript demonstrates balancing an equation with five different elements and parentheses.
A criss-cross multiplication method is introduced for balancing hydrogen in a compound.
The transcript concludes with balancing an equation with nitrogen, copper, and oxygen.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: