Wavelength, Frequency, Energy, Speed, Amplitude, Period Equations & Formulas - Chemistry & Physics
TLDRThis video delves into the characteristics of waves, explaining amplitude, wavelength, frequency, period, and energy. It demonstrates how to calculate these properties using graphs and equations. The relationship between wavelength and frequency, and how it changes when light travels through different media, is also discussed. The video concludes with an overview of the electromagnetic spectrum, detailing the order from longest wavelength to shortest and the corresponding energy levels.
Takeaways
- 🌊 Waves have characteristics like frequency, wavelength, energy, amplitude, and speed.
- 📈 Amplitude is the height from the center to the peak (crest) or trough of a wave, and can be calculated as half the distance between the highest and lowest points.
- 🌀 Wavelength is the distance of one complete cycle of a wave, measured from one peak or trough to the next.
- 🔄 The period is the time taken for one complete cycle of a wave, while frequency is the number of cycles per second (inversely related to period).
- 📊 To find the period from a graph, divide the total time by the number of cycles, and for frequency, divide the number of cycles by the total time.
- 🌌 The speed of light (C) in a vacuum is a constant at 3 * 10^8 m/s, and is used to relate wavelength (λ) and frequency (f) through the equation C = λf.
- 💡 Energy of a photon can be calculated using Planck's constant (h) multiplied by the frequency (E = h * f), and is measured in joules (J) or electron volts (eV).
- 🔄 When light travels through different materials, its speed and wavelength change, but its frequency remains constant.
- 🌍 The index of refraction (n) affects the speed (V = C / n) and wavelength (λ_n = λ / n) of light in a material, where n is the refractive index.
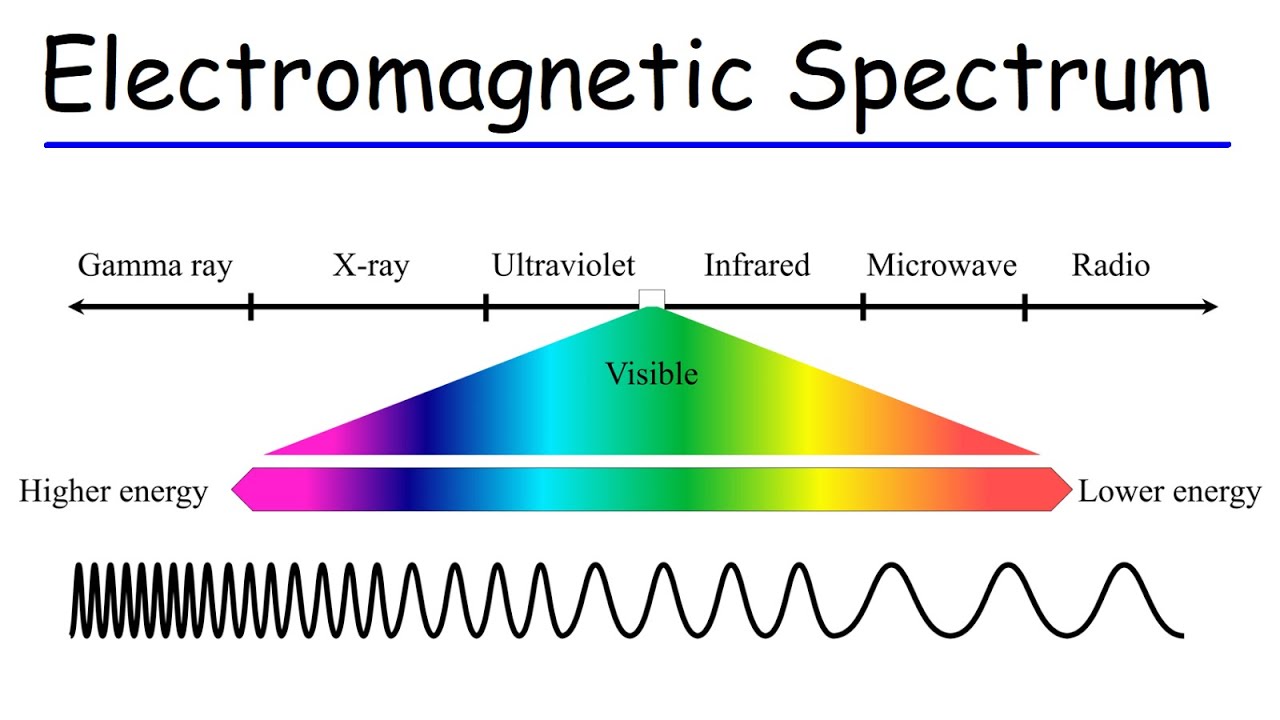
- 🌈 The electromagnetic spectrum is ordered from longest wavelength (low energy) to shortest wavelength (high energy): radio waves, microwaves, infrared, visible light, ultraviolet, X-rays, gamma rays, and cosmic radiation.
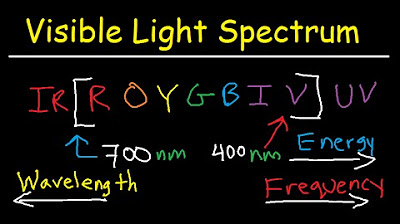
- 📏 The visible light spectrum ranges from about 400 nm (violet) to 700 nm (red), with each color having a specific wavelength range.
Q & A
What are the key characteristics of waves discussed in the video?
-The key characteristics of waves discussed in the video include frequency, wavelength, energy, amplitude, speed, and period.
How is the amplitude of a wave calculated?
-The amplitude of a wave is calculated as the height between the center and the peak (crest) or the depth to the trough. It can also be calculated by subtracting the lower value from the higher value and dividing by two, which gives the half-difference between the peak and trough.
What is the definition of wavelength?
-Wavelength is the length of one complete cycle of a wave, measured as the distance between two consecutive points in phase, such as from one crest to the next crest or one trough to the next trough.
How can you determine the frequency and period of a wave?
-Frequency is the number of cycles a wave completes in one second and is the inverse of the period. The period is the time it takes for the wave to complete one full cycle. To calculate the frequency from a graph, you divide the number of cycles by the time, and for the period, you divide the time by the number of cycles.
What is the relationship between wavelength and frequency?
-Wavelength and frequency are inversely related. As the wavelength increases, the frequency decreases, and vice versa. This relationship can be expressed by the equation C = Lambda * f, where C is the speed of light, Lambda is the wavelength, and f is the frequency.
How does the speed of light change when it passes through different materials?
-The speed of light changes when it passes through different materials due to the change in the index of refraction. The speed of light in a material is given by the equation V = C / n, where V is the speed of light in the material, C is the speed of light in a vacuum, and n is the index of refraction of the material.
What happens to the wavelength of light when it passes from one medium to another?
-When light passes from one medium to another, its wavelength changes inversely with the index of refraction of the new medium. If the index of refraction increases, the wavelength decreases, and if the index of refraction decreases, the wavelength increases.
How is the energy of a photon related to its frequency?
-The energy of a photon is directly proportional to its frequency. This relationship is given by the equation E = h * f, where E is the energy of the photon, h is Planck's constant, and f is the frequency of the photon.
What is the significance of the electromagnetic spectrum in understanding wave characteristics?
-The electromagnetic spectrum organizes different types of electromagnetic waves by their wavelength or frequency. It ranges from long-wavelength, low-frequency, low-energy radio waves to short-wavelength, high-frequency, high-energy gamma rays. Understanding the electromagnetic spectrum helps in comprehending the behavior and interaction of waves with different materials and their applications.
What are the typical wavelengths associated with visible light spectrum colors?
-The typical wavelengths for colors in the visible light spectrum are approximately 700 nm for red light, 600-620 nm for orange light, 570-590 nm for yellow light, 500-570 nm for green light, 450-500 nm for blue light, and 400-450 nm for violet light.
How does the index of refraction affect the speed and wavelength of light when it transitions between air and water?
-When light transitions from air to water, the index of refraction increases, causing the speed of light to decrease and the wavelength to shorten. The frequency, however, remains unchanged because it is a property of the wave source and does not depend on the medium through which the wave is traveling.
Outlines
🌊 Understanding Wave Characteristics
This paragraph introduces the fundamental characteristics of waves, such as frequency, wavelength, energy, amplitude, speed, and period. It explains how to calculate amplitude from a graph by finding the height from the center to the peak (crest) or by taking half the difference between the highest and lowest values. The wavelength is defined as the length of one complete cycle, and an example is provided to illustrate how to measure it from a graph. The paragraph emphasizes the importance of these wave properties in understanding wave behavior.
📊 Calculating Amplitude and Wavelength
The focus of this paragraph is on the methods to calculate amplitude and wavelength from graphical representations of waves. It provides a detailed example where the amplitude is determined by finding the midpoint between the highest value and the lowest value (trough), and then calculating the distance between this midpoint and the trough or the crest. The wavelength is calculated by determining the length of one complete cycle and then using the ratio of the total distance to the number of cycles. The paragraph further explains the relationship between frequency and period, stating that frequency is the inverse of the period, and provides examples of how to calculate these from a graph.
🔄 Frequency and Wavelength Relationship
This paragraph delves into the inverse relationship between wavelength and frequency. It explains that as the wavelength increases, the frequency decreases, and vice versa. The paragraph also introduces the concept that the speed of light (C) is equal to the product of wavelength (Lambda) and frequency (f), highlighting that this relationship is consistent regardless of the medium through which light travels. The discussion includes the impact of different materials on the speed of light and wavelength, with the frequency remaining constant. The paragraph provides an example of how to calculate the frequency of a photon in a vacuum and its energy, using Planck's constant. It also touches on how the speed and wavelength of light change when it passes from one medium to another, such as from water to glass.
💡 The Electromagnetic Spectrum
The paragraph concludes with an overview of the electromagnetic spectrum, ranging from long wavelengths and low energy (radio waves) to short wavelengths and high energy (cosmic radiation). It explains the order of the spectrum from radio waves to microwaves, infrared, visible light, ultraviolet, X-rays, gamma rays, and cosmic radiation. The paragraph emphasizes the direct relationship between frequency and energy, with higher frequencies corresponding to higher energy. It also discusses the visible light spectrum, detailing the wavelength ranges for red, orange, yellow, green, blue, indigo, and violet light. The explanation includes how the wavelength and energy of light change as it transitions from one medium to another, such as from diamond to glass, and concludes with a summary of the visible light spectrum's color ranges.
Mindmap
Keywords
💡Wavelength
💡Amplitude
💡Frequency
💡Period
💡Speed
💡Energy
💡Index of Refraction
💡电磁谱 (Electromagnetic Spectrum)
💡Refraction
💡Visible Light
Highlights
The video discusses the characteristics of waves, including frequency, wavelength, energy, amplitude, speed, and period.
Amplitude is the height between the center and the peak or trough of a wave, and can be calculated by subtracting the bottom value from the top value, then dividing by two.
Wavelength is the length of one cycle of a wave, and is calculated by dividing the total distance by the number of cycles.
Frequency is the number of cycles a wave makes in a single second and is the inverse of the period.
The period is the time it takes for a wave to complete one cycle, calculated by dividing the time by the number of cycles.
The speed of light in a vacuum is 3 * 10^8 m/s, and this speed is used to calculate the frequency of a photon in pure empty space.
The energy of a photon can be calculated using the equation E = Planck's constant * frequency, with Planck's constant being 6.626 * 10^-34 Js.
When light passes through a material, its speed and wavelength change, but its frequency remains the same.
The index of refraction affects the speed of light and wavelength in a material, with higher indices resulting in slower speeds and shorter wavelengths.
The electromagnetic spectrum is ordered from long wavelength to short wavelength, or from low energy to high energy.
The visible light spectrum ranges from red light at approximately 700 nm to violet light at about 400 nm.
As one moves through the electromagnetic spectrum from left to right, both frequency and energy increase.
The relationship between wavelength and frequency is given by the equation C = Lambda * f, where C is the speed of light, Lambda is the wavelength, and f is the frequency.
The speed of light in a material is calculated using the equation V = C / n, where n is the index of refraction.
The energy of a photon can be converted to electron volts using the conversion factor of 1 eV = 1.62 * 10^-19 J.
The video provides practical examples and methods for calculating wave properties from graphs and equations.
The video concludes with a comprehensive overview of the visible light spectrum and its corresponding wavelengths.
Transcripts
Browse More Related Video
Electromagnetic Spectrum - Basic Introduction

Electromagnetic Spectrum Explained - Gamma X rays Microwaves Infrared Radio Waves UV Visble Light
Visible Light Spectrum Explained - Wavelength Range / Color Chart Diagram - Chemistry
Electromagnetic Waves

Waves 1: Wave Characteristics

Lecture 6 - Wave Theory
5.0 / 5 (0 votes)
Thanks for rating: