The Limiting Reactant Question That's Found on Most Final Exams | Study Chemistry With Us
TLDRIn this educational video transcript, the instructor guides the student through chemistry practice problems, focusing on balancing chemical equations and understanding the concept of limiting reactants. They work through a step-by-step process, converting grams to moles and vice versa, to determine the theoretical and actual yields of a reaction, and finally calculate the percent yield. The conversational tone and detailed explanations aim to demystify the topic, emphasizing the importance of these calculations in chemistry.
Takeaways
- 📚 The script is a dialogue focused on chemistry practice problems, specifically balancing chemical equations and determining limiting reactants.
- 🔍 The conversation includes a step-by-step walkthrough of balancing equations and understanding the process of converting between grams and moles in chemical reactions.
- 🧪 The importance of using the periodic table and molar masses is emphasized for solving stoichiometry problems.
- 📉 Stress is expressed about upcoming exams and the desire to maintain a high grade, indicating the pressure students may feel in academic settings.
- 📝 The process of finding the limiting reactant involves converting the mass of each reactant to moles and then using the balanced chemical equation to find the theoretical yield of the product.
- ⚖️ The script discusses the concept of theoretical yield and how it is calculated using the limiting reactant.
- 🔄 The steps for converting between grams and moles include using molar masses and coefficients from the balanced chemical equation to find the moles of products.
- 📉 The script highlights the significance of limiting reactants in determining the maximum amount of product that can be formed in a chemical reaction.
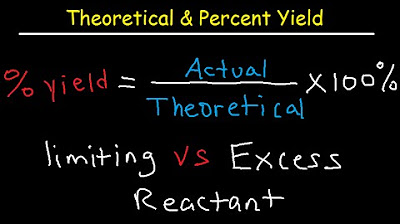
- 📊 The calculation of percent yield is mentioned as an important concept, which is the ratio of actual yield to theoretical yield, expressed as a percentage.
- 📚 The dialogue suggests that the topics covered are likely to appear on upcoming exams, including finals, underscoring their importance in the chemistry curriculum.
- 🔑 The script ends with advice to review notes, watch additional videos, and practice more to fully understand and be prepared for exams on these topics.
Q & A
What is the first step in solving the chemistry problem presented in the transcript?
-The first step is to balance the chemical equation by multiplying the reactants and products as needed to ensure that the number of atoms of each element is the same on both sides of the equation.
Why is it necessary to multiply sodium nitrate by 2 in the given chemical equation?
-It is necessary to multiply sodium nitrate by 2 to balance the nitrate ions (NO3-) in the equation, as each nitrate ion has a -1 charge and there are three of them in the product side.
What is the molar mass of copper(II) chloride (CuCl2) used in the transcript?
-The molar mass of copper(II) chloride is calculated by adding the molar mass of copper (63.55) and twice the molar mass of chlorine (2 x 35.45), which gives approximately 134.45 grams per mole.
How does the process of finding the limiting reactant relate to the concept of molar mass?
-The process of finding the limiting reactant involves converting the given mass of each reactant to moles using their respective molar masses, then using the balanced chemical equation to determine which reactant will be completely consumed first, thus limiting the amount of product that can be formed.
What is the significance of the balanced equation coefficients in calculating moles of products from reactants?
-The coefficients in a balanced equation represent the ratio of moles of reactants and products. They are used to calculate the moles of products that can be formed from a given amount of reactants.
Why is it important to convert grams to moles and then back to grams in the process described in the transcript?
-This conversion process is important because it allows chemists to compare the amounts of different substances in a reaction based on their molar quantities, which is essential for determining the limiting reactant and the theoretical yield of a reaction.
What does the term 'theoretical yield' refer to in the context of the chemistry problem?
-The theoretical yield refers to the maximum amount of product that can be produced from a given amount of reactants, assuming 100% efficiency and no side reactions.
How is the percent yield calculated in a chemical reaction?
-The percent yield is calculated by dividing the actual yield (the amount of product actually obtained) by the theoretical yield (the maximum amount of product that could be produced) and then multiplying by 100 to get a percentage.
What is the purpose of finding the excess reactant after the reaction?
-Finding the excess reactant helps determine how much of the reactant is left unreacted after the reaction has occurred, which is useful for understanding the efficiency of the reaction and for planning subsequent reactions.
Why is the limiting reactant concept considered so important in chemistry?
-The limiting reactant concept is important because it is fundamental to understanding how much product can be formed in a chemical reaction, which is crucial for stoichiometry calculations, efficiency analysis, and planning chemical processes in industries and laboratories.
Outlines
🧪 Chemistry Practice Problems Session
The script begins with a chemistry practice session where the participants are working through chemical equation balancing and stoichiometry problems. They discuss the process of balancing equations, converting between grams and moles, and understanding the charges of ions. The conversation is casual, with one participant expressing stress and the other guiding them through the steps, including using the periodic table and flashcards for reference.
📚 Studying for a Chemistry Exam
This paragraph focuses on the preparation for a chemistry exam. The participants discuss the importance of understanding concepts rather than memorizing them, especially for topics like limiting reactants and percent yield. There's a detailed explanation of how to find the limiting reactant by using the mass of reactants and converting it to the mass of the product, in this case, sodium chloride. The conversation includes steps like writing balanced equations, converting grams to moles, and using molar masses.
🔍 Detailed Calculation Process for Limiting Reactants
The script continues with a detailed walkthrough of calculating the limiting reactant in a chemical reaction. It involves determining the molar mass of reactants, converting grams to moles, and using the balanced chemical equation to find the moles of product that can be formed. The participants carefully explain each step, including the importance of canceling units when performing conversions, and emphasize the need to understand the process rather than just memorizing it.
📝 Step-by-Step Guide to Chemical Conversions
The participants provide a step-by-step guide on how to convert between grams and moles for chemical compounds, specifically focusing on copper chloride and sodium chloride. They explain the process of using molar masses and balanced equations to find the relationship between reactants and products. The conversation includes practical tips, such as writing out the full molar mass without rounding and ensuring units cancel out correctly.
🔢 Calculating Theoretical and Actual Yields
This section delves into the calculations of theoretical and actual yields in a chemical reaction. The participants explain how to find the theoretical yield using the limiting reactant and then calculate the actual yield based on the given data. They discuss the importance of significant figures and rounding in scientific calculations, and the need to understand the difference between theoretical and actual yields.
📉 Determining the Excess Reactant
The script explains how to determine the excess reactant in a chemical reaction after identifying the limiting reactant. The participants guide through the process of subtracting the amount of reactant used from the original amount to find the leftover reactant. They emphasize the importance of understanding this concept, as it is a common topic in chemistry exams.
📈 Calculating Percent Yield
The participants discuss the concept of percent yield in chemistry, which is a measure of the efficiency of a chemical reaction. They explain the formula for calculating percent yield and stress the importance of using the theoretical yield and actual yield in the calculation. The conversation includes a reminder to double-check calculations and the significance of understanding the process for exams.
🎓 Importance of Understanding Limiting Reactants
The script concludes with an emphasis on the importance of understanding limiting reactants, which is highlighted as a key topic in chemistry. The participants predict that this topic will likely appear on upcoming exams and finals, and they encourage practice and review of related materials, such as study plan videos and notes on limiting reactants and percent yield.
📚 Encouragement for Continued Practice
The final paragraph encourages continued practice with chemistry problems, especially for those who are finding the concepts challenging. The participants acknowledge that everyone is learning and that it's okay to struggle with new material. They suggest watching additional videos and reviewing notes to gain a better understanding of the topics.
Mindmap
Keywords
💡Limiting Reactant
💡Molar Mass
💡Balanced Equation
💡Stoichiometry
💡Theoretical Yield
💡Actual Yield
💡Percent Yield
💡Moles
💡Coefficients
💡Excess Reactant
Highlights
The transcript features a step-by-step walkthrough of chemistry practice problems, focusing on balancing chemical equations and identifying limiting reactants.
The discussion includes a methodical approach to converting grams to moles and vice versa, which is crucial for solving stoichiometry problems.
Stress management is subtly addressed, as the speaker acknowledges feeling stressed and encourages maintaining a calm approach to problem-solving.
The use of the periodic table as a reference tool is emphasized, highlighting its importance in chemistry for determining atomic masses and charges.
The transcript demonstrates the application of molar mass in calculations, a fundamental concept in chemistry for determining the amount of substance.
The concept of theoretical yield is introduced, which is essential for understanding the maximum amount of product that can be formed in a chemical reaction.
The process of finding the limiting reactant is thoroughly explained, which is a key step in determining which reactant will be completely consumed in a reaction.
The transcript emphasizes the importance of understanding the balance equation coefficients for converting between moles of different substances in a reaction.
The practical application of stoichiometry is showcased through the calculation of grams of sodium chloride (NaCl) produced from given reactants.
The concept of excess reactant and how to calculate the leftover amount after the reaction is thoroughly discussed.
The transcript provides guidance on setting up and solving for percent yield, a critical measure of the efficiency of a chemical reaction.
The importance of significant figures in scientific calculations is touched upon, ensuring accuracy and precision in results.
The transcript includes a reminder to always check calculations and understand the process, rather than just memorizing steps.
The speaker shares personal experiences and strategies for learning, such as using flashcards and rewriting notes for better retention.
The transcript concludes with advice on further study, including revisiting videos and notes, to solidify understanding of limiting reactants and percent yield.
The significance of limiting reactants in chemistry exams and finals is highlighted, with the speaker predicting its appearance on future assessments.
The transcript offers a candid look at the learning process, showing that even experienced individuals can struggle with new concepts and need practice.
Transcripts
Browse More Related Video

Stoichiometry: Limiting Reactant, Left Over Excess Reactant, Percent Yield | Study Chemistry With Us

Most Common Chemistry Final Exam Question: Limiting Reactants Review
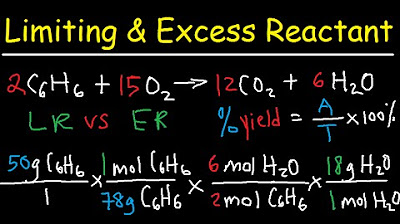
Stoichiometry - Limiting & Excess Reactant, Theoretical & Percent Yield - Chemistry

Practice Problem: Limiting Reagent and Percent Yield

How to calculate Theoretical Yield and Percent Yield?
Theoretical, Actual, Percent Yield & Error - Limiting Reagent and Excess Reactant That Remains
5.0 / 5 (0 votes)
Thanks for rating: