Significant Figures Step by Step | How to Pass Chemistry
TLDRIn this video, Melissa Maribel explains six rules for determining significant figures, or sig figs, which help gauge the accuracy of measurements. Key rules include: non-zero numbers and zeros between them are significant, zeros after a decimal are significant, leading zeros are not, and zeros in large numbers without a decimal are not significant. For addition and subtraction, focus on the least decimal places; for multiplication and division, consider the least sig figs. Melissa provides examples and practice problems to reinforce these concepts, aiming to help students master Chemistry with ease.
Takeaways
- 🔢 Significant figures (sig figs) are the digits in a number that indicate its precision and accuracy.
- 💡 Rule 1: All non-zero digits are considered significant.
- 🌟 Rule 2: Zeros between non-zero digits are significant.
- 📉 Rule 3: Zeros after a decimal point are significant.
- 🌌 Rule 4: Zeros in scientific notation are significant.
- ❌ Rule 5: Leading zeros are not significant.
- 🔍 Rule 6: Zeros in large numbers without a decimal point are not significant.
- ➕ When adding or subtracting, align the decimal points and round to the least number of decimal places.
- ✂️ For multiplication and division, round to the number with the fewest significant figures.
- 📚 Practice is key; the video offers additional practice problems and step-by-step solutions.
- 👍 Encouragement to like and subscribe for more educational content.
- 🎓 The channel aims to help students succeed in Chemistry.
Q & A
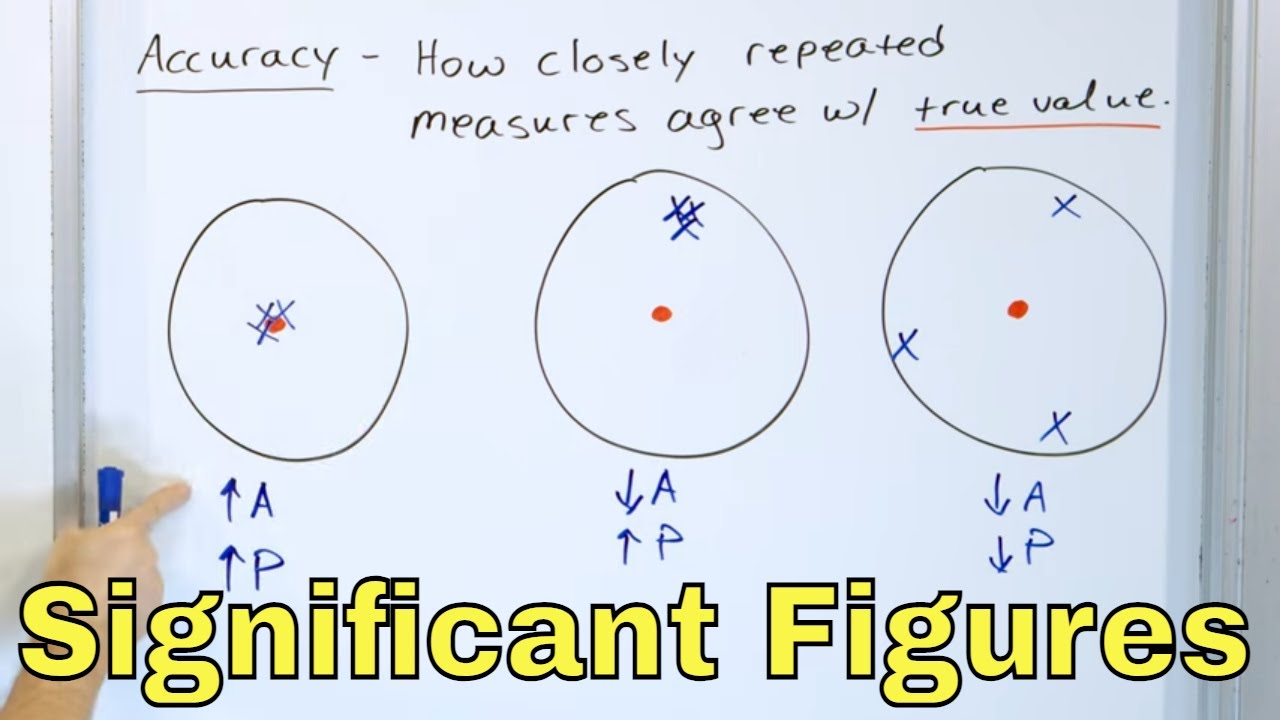
What are significant figures and why are they important?
-Significant figures, or sig figs, are the digits within a number that help determine the accuracy of a data value or measurement. They are important because they indicate the precision of the measurement and prevent misrepresentation of data.
What is the first rule for determining significant figures in a number?
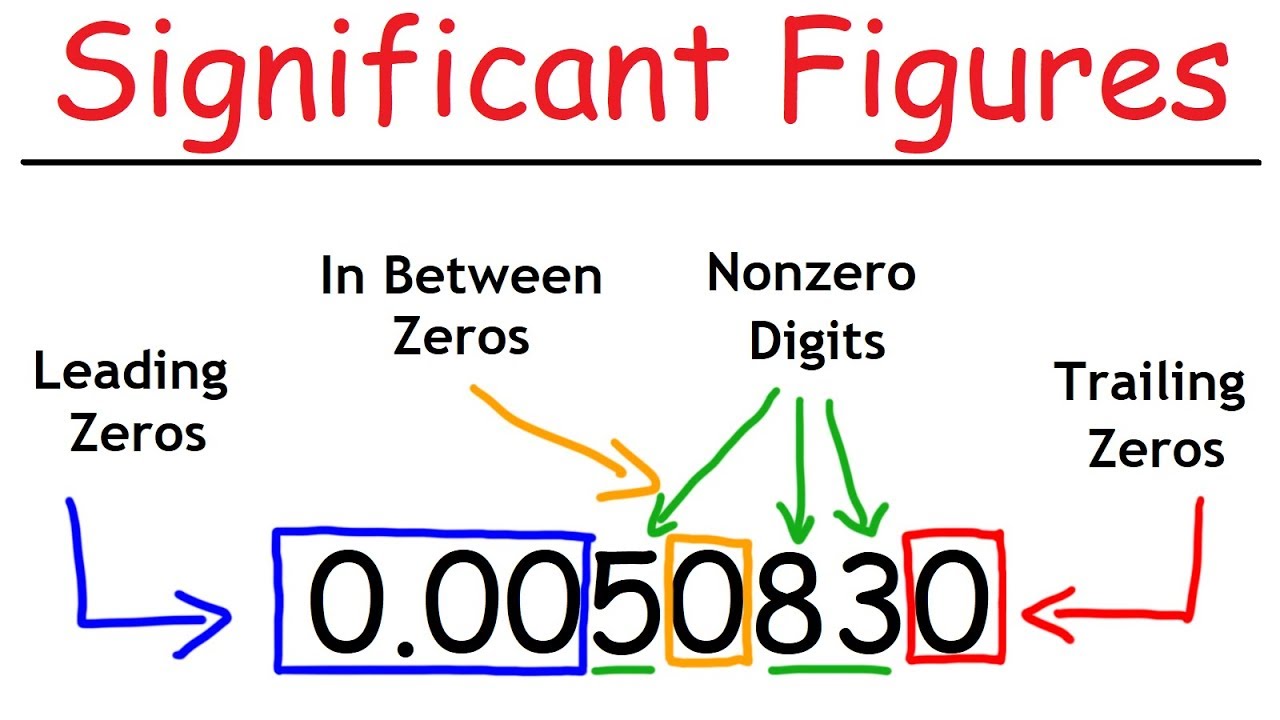
-The first rule states that all non-zero numbers are significant. Every digit in a non-zero number is considered significant.
How do you treat zeros that are in the middle of non-zero numbers?
-Any zeros that are in the middle of non-zero numbers are significant. For example, in the number 5208, the zeros between the 5 and the 8 are significant.
Are zeros after the decimal point always significant?
-Yes, zeros after the decimal point are always significant. They indicate precision up to a certain place value.
How are zeros treated in scientific notation?
-In scientific notation, any zeros that are part of the coefficient (the number before the multiplication sign) are significant.
What about leading or beginning zeros in a number, are they significant?
-Leading or beginning zeros in a number are not significant. They are placeholders and do not contribute to the accuracy of the measurement.
How do you determine the significance of zeros in a large number without a decimal?
-Zeros in a large number without a decimal are not significant. Only the non-zero digits count towards the significant figures.
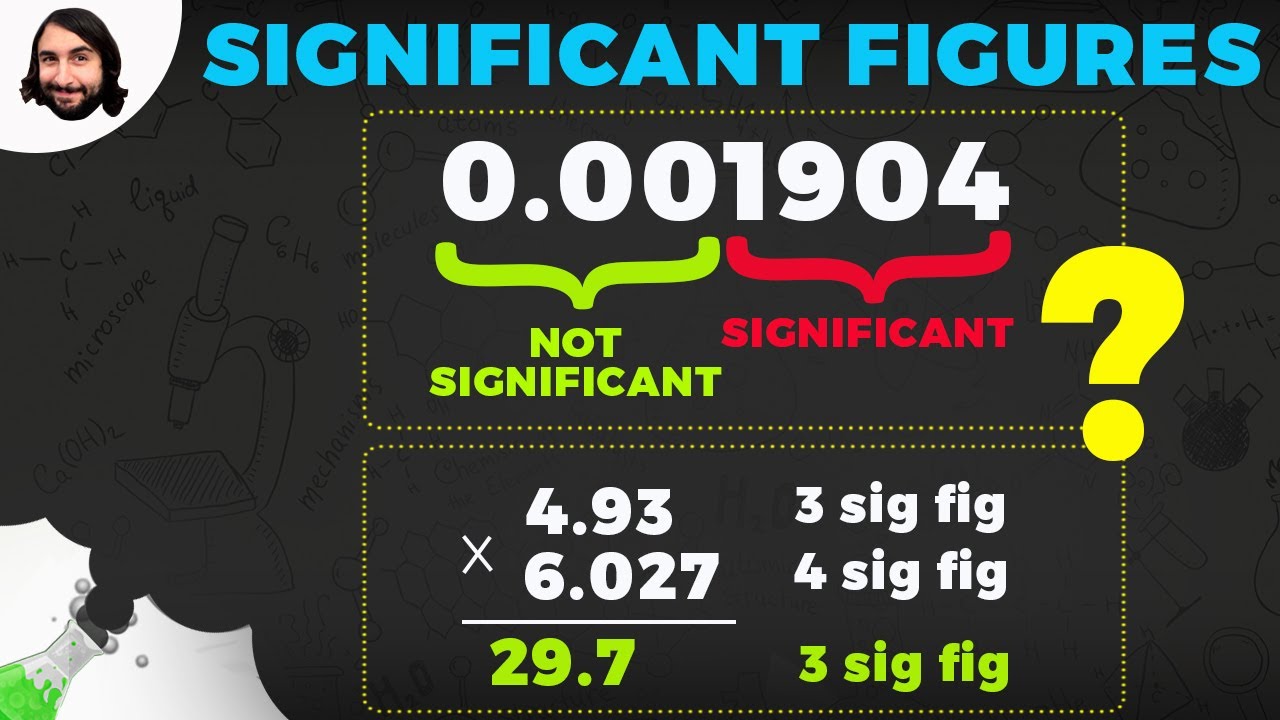
When adding and subtracting numbers, what determines the number of significant figures in the result?
-When adding and subtracting, you look for the least amount of decimal places among the numbers involved. The result should be rounded to the same number of decimal places as the number with the least.
In multiplication and division, how do you determine the number of significant figures in the result?
-When multiplying and dividing, you look for the least amount of significant figures among the numbers involved. The result should be rounded to the same number of significant figures as the number with the least.
What should you do if there is a zero in the last place of a number after multiplication?
-If there is a zero in the last place after multiplication, and there is no decimal, you should consider the significant figures based on the non-zero digits and ignore the trailing zero.
Where can I find additional practice problems and step-by-step answers related to significant figures?
-Additional practice problems and step-by-step answers can be found in the description box of the video, where a link is provided.
Outlines
📚 Understanding Significant Figures
In this educational video, Melissa Maribel introduces the concept of significant figures and their importance in determining the accuracy of data values or measurements. She explains six key rules for identifying significant figures, including the significance of non-zero numbers, zeros between non-zero digits, zeros after a decimal point, and zeros in scientific notation. She also clarifies that leading zeros and zeros within large numbers without a decimal are not significant. Additionally, Melissa provides guidance on how to handle significant figures during arithmetic operations like addition, subtraction, multiplication, and division, emphasizing the importance of rounding to the correct number of significant figures. The video concludes with an invitation for viewers to practice with provided problems and to subscribe for more educational content.
🎥 Behind-the-Scenes Bloopers
This section captures a light-hearted moment from the video production, where Melissa Maribel is interrupted by the cameraman regarding her hair. She playfully denies any action and thanks a 'random hand' for assistance, adding a touch of humor to the educational content. The blooper ends with Melissa laughing and flipping her hair, showcasing the casual and friendly tone of the video series.
Mindmap
Keywords
💡Significant Figures
💡Decimal Place
💡Scientific Notation
💡Leading Zeros
💡Large Number
💡Rounding
💡Multiplication
💡Division
💡Practice Problems
💡Accuracy
💡Chemistry
Highlights
Significant figures determine the accuracy of a data value or measurement.
All non-zero numbers are significant.
Zeros between non-zero numbers are significant.
Zeros after the decimal point are significant.
Zeros in scientific notation are significant.
Leading zeros are not significant.
Zeros in large numbers without a decimal are not significant.
When adding and subtracting, find the least amount of decimal places.
Align decimals when adding and rounding to the least decimal place.
Rounding involves looking at the number to the right of the rounding place.
When multiplying and dividing, find the least amount of significant figures.
Count significant figures in each number before multiplication or division.
After multiplying, remove unnecessary decimal places.
Practice problems are available in the description box.
A recap of significant figures and their rules is provided.
The importance of understanding significant figures for accuracy in Chemistry.
Engagement with the audience to provide help in Chemistry.
A humorous moment with a hair flip and a random hand.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: