Bohr’s Atomic Model | Atoms and Molecules | Infinity Learn NEET
TLDRThis script explores the evolution of atomic models, highlighting Rutherford's limitations and Neils Bohr's refinements, introducing the concept of discrete electron orbits without energy radiation. It visually compares atomic structure to our solar system, explains electron shells or energy levels, and introduces the discovery of the neutron by James Chadwick, completing the atom's triad of subatomic particles. The summary leaves curiosity about electron distribution in orbitals for future exploration.
Takeaways
- 🌟 Rutherford's atomic model was foundational in explaining atomic structure but faced theoretical issues with the stability of atoms due to the expected energy loss from accelerating electrons.
- 🔁 The classical problem of electrons radiating energy and spiraling into the nucleus was resolved by Neils Bohr's modifications to the atomic model.
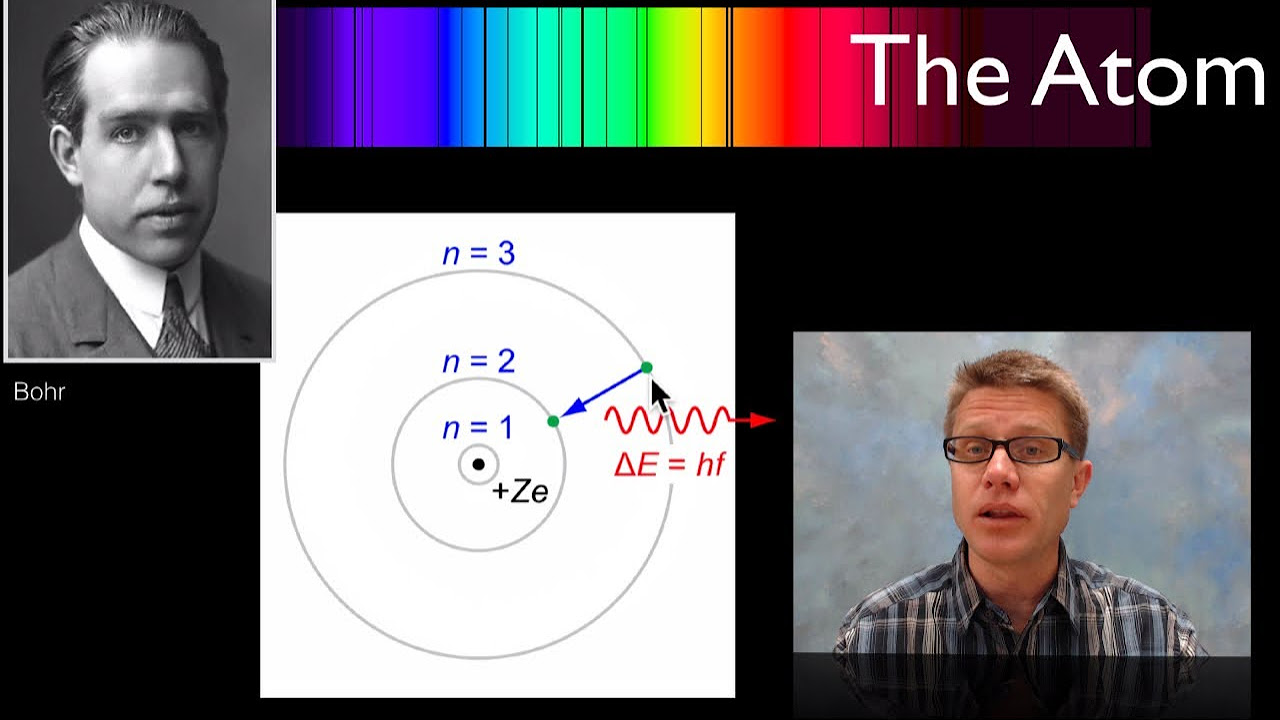
- 📍 Bohr proposed that electrons occupy discrete orbits where they do not radiate energy, thus maintaining atomic stability.
- 🌍 The atomic structure is compared to the solar system, with the nucleus as the sun and electrons as planets orbiting in fixed paths.
- 🔶 Electrons are found in shells or energy levels, which are defined by their distance from the nucleus and their energy states.
- 👉 Bohr's model introduced the concept of quantization, where electrons can only exist in certain allowed energy levels without radiating energy.
- 🔢 The shells are named alphabetically (K, L, M, N, etc.) or numerically (n=1, n=2, n=3, etc.), indicating their position relative to the nucleus.
- 🚀 The discovery of the neutron by Sir James Chadwick in 1932 completed the picture of the atom, which consists of protons, neutrons, and electrons.
- ⚛️ Neutrons are neutral particles with a mass similar to that of protons, and they reside in the nucleus alongside protons.
- 🧠 The distribution of electrons in orbitals and the maximum number of electrons per orbital is a topic for further exploration, hinting at upcoming content.
- 🔑 The script suggests that understanding the atomic structure is not complete without knowing the arrangement and capacity of electrons in the orbitals.
Q & A
What was the main issue with Rutherford's atomic model?
-Rutherford's model suggested that electrons orbit the nucleus like planets around the sun, but it failed to explain why electrons do not spiral into the nucleus due to the continuous loss of energy as they radiate while revolving.
Why do electrons not radiate energy while orbiting in the Rutherford model?
-According to classical physics, electrons should radiate energy while in circular motion, leading to instability. However, in nature, atoms are stable, indicating that Rutherford's model needed modification, which was provided by Neils Bohr.
What was Neils Bohr's contribution to the atomic model?
-Bohr introduced the concept of quantized orbits or energy levels where electrons do not radiate energy while orbiting. This explained the stability of atoms.
What are the discrete orbits referred to as in Bohr's model?
-In Bohr's model, these discrete orbits are referred to as shells or energy levels, which are quantized and do not allow for energy radiation by the electrons.
How are the electron orbitals or shells named in the atomic structure?
-The electron orbitals are named alphabetically starting from the nucleus as 'K shell', 'L shell', 'M shell', 'N shell', and so on, or numerically as 'n equals 1', 'n equals 2', 'n equals 3', etc.
What is the significance of the term 'energy levels' in the context of Bohr's atomic model?
-The term 'energy levels' signifies that each shell or orbital has a defined energy state, and electrons do not radiate energy while in these states, maintaining the atom's stability.
Can the shells or energy levels be represented numerically?
-Yes, the shells or energy levels can be represented numerically using the principal quantum number 'n', starting with n=1 for the innermost shell and increasing with distance from the nucleus.
What is the role of the nucleus in the atomic structure?
-The nucleus serves as the central point of the atom, containing positively charged protons and neutral neutrons, around which the negatively charged electrons orbit in fixed orbitals.
Who discovered the neutron and in what year?
-Sir James Chadwick discovered the neutron in 1932, identifying it as a neutral particle with a mass almost equivalent to that of a proton.
How does the discovery of the neutron contribute to the understanding of atomic structure?
-The discovery of the neutron, a neutral particle in the nucleus, completed the basic model of the atom, which consists of a nucleus with protons and neutrons, and electrons orbiting in fixed energy levels.
How are electrons distributed in the orbitals, and is there a pattern to this distribution?
-The distribution of electrons in orbitals follows the principles of quantum mechanics, including the Pauli exclusion principle and Hund's rule, which will be explained in upcoming videos.
Outlines
🔬 Rutherford's Atomic Model and Its Limitations
This paragraph discusses Rutherford's atomic model, which was successful in explaining the structure of atoms but theoretically inadequate. It highlights the issue of electrons in circular orbits gaining acceleration and radiating energy, leading to instability. The paragraph introduces Neils Bohr, who provided modifications to the model, including the concept of discrete orbits where electrons do not radiate energy, thus explaining atomic stability.
🌌 Bohr's Postulates on Atomic Structure
The paragraph delves into Bohr's atomic model, emphasizing the existence of fixed, non-radiative electron orbits or shells. It compares the atomic structure to our solar system, with the nucleus at the center and electrons in defined orbitals. The naming convention for these shells, both alphabetically (K, L, M, N, etc.) and numerically (n=1, n=2, etc.), is explained, as well as the presence of a third subatomic particle, the neutron, discovered by James Chadwick in 1932.
Mindmap
Keywords
💡Rutherford's atomic model
💡acceleration
💡Neils Bohr
💡discrete orbits
💡electron
💡nucleus
💡energy levels
💡shells or energy levels
💡K shell
💡neutron
💡quantum mechanics
Highlights
Rutherford's atomic model was commendable but theoretically inappropriate due to the instability it predicted.
Charged objects in circular motion gain acceleration and should radiate energy, contradicting the stability of atoms.
Electrons in Rutherford's model would lose energy and fall into the nucleus, which contradicts atomic stability.
Neils Bohr introduced modifications to Rutherford's model to explain atomic stability.
Bohr proposed that electrons occupy discrete orbits where they do not radiate energy.
Electrons' paths or orbits are fixed and defined, similar to the solar system's planetary motion.
Electron orbitals are referred to as shells or energy levels, indicating defined energy states.
Shells are named alphabetically (K, L, M, N, ...) or numbered (n=1, n=2, n=3, ...).
The nucleus contains protons and neutrons, with electrons revolving in fixed orbitals.
Sir James Chadwick discovered the neutron, a neutral subatomic particle with mass similar to a proton.
The complete atomic design includes a nucleus with protons and neutrons, and electrons in fixed orbitals.
The distribution of electrons in orbitals and the maximum number of electrons per orbital will be explored in upcoming content.
Bohr's model explains the stability of atoms by introducing quantized energy levels.
Electrons in different shells have distinct energy levels, contributing to atomic stability.
The atomic structure's understanding is fundamental to grasping chemical and physical properties of elements.
Further exploration of electron distribution will provide insights into atomic structure complexity.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: