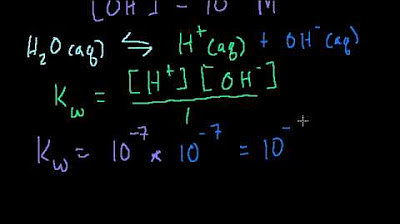pH, pOH of strong acids and bases | Chemistry | Khan Academy
TLDRThe video script discusses the concept of water autoionization and the equilibrium between hydronium and hydroxide ions. It explains the equilibrium constant (Kw) and its temperature dependence, leading to the definition of pH and pOH. The script uses the example of adding hydrochloric acid to water to demonstrate how pH changes with the introduction of an acid, resulting in a pH of 0 for a 1 M HCl solution. Conversely, adding a strong base like potassium hydroxide results in a pH of 14 and a pOH of 0 for the same concentration. The video emphasizes the importance of understanding pH and pOH in relation to acid-base reactions and their practical implications.
Takeaways
- 🌊 Water in its autoionized equilibrium state produces hydrogen and hydroxide ions, primarily in the form of hydronium (H3O+).
- 📈 At 25°C, the equilibrium constant (Kw) for water is 10^-14, with hydrogen and hydroxide ion concentrations both at 10^-7 M, resulting in a pH of 7 for pure water.
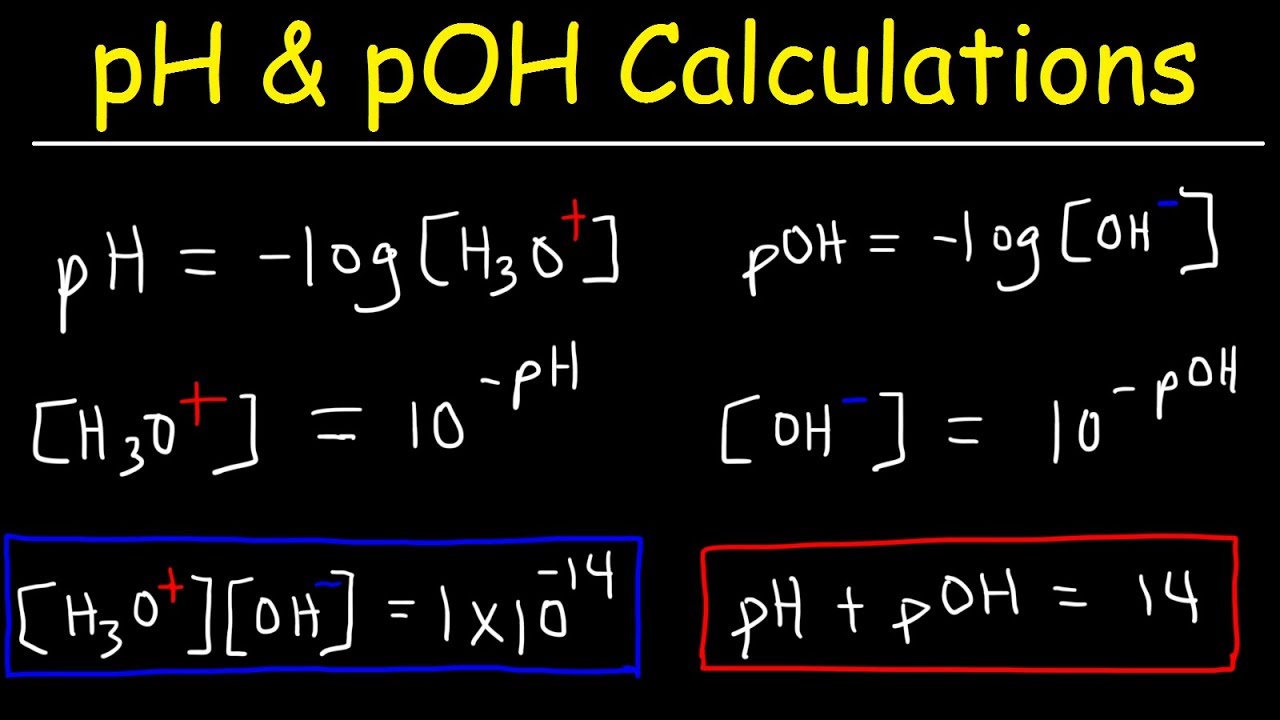
- 📚 The pH is calculated as the negative logarithm (base 10) of the hydrogen ion concentration, and the pOH is the negative logarithm of the hydroxide ion concentration.
- ⚖️ The pKw, which is 14 at 25°C, is a constant value that represents the sum of the pH and pOH of a solution, reflecting the self-ionization of water.
- 💧 Adding a strong acid like hydrochloric acid (HCl) to water increases the hydrogen ion concentration, resulting in a lower pH value (0 in a 1 M HCl solution).
- 🔄 Le Chatelier's Principle suggests that adding a strong acid to water will shift the equilibrium to consume more hydroxide ions, significantly lowering their concentration.
- 🧪 A 1 M solution of a strong base like potassium hydroxide (KOH) will have a pH of 14 and a pOH of 0 due to complete dissociation in water.
- 🔢 The pH scale is not absolute; solutions can have pH values lower than 0 or higher than 14 when dealing with very strong acids or bases.
- 📊 The logarithmic nature of the pH and pOH scales means that a tenfold change in concentration results in a unit change in pH or pOH (e.g., 10 M HCl results in a pH of -1).
- 🚫 Be cautious with strong acids or bases, as they can be harmful; a pH of 0 indicates a highly acidic solution, which can be dangerous.
Q & A
What is the autoionization of water and what products does it form?
-The autoionization of water refers to the process where water dissociates into its ions, forming hydrogen ions (protons) and hydroxide ions. These hydrogen ions often attach to other water molecules to form hydronium ions (H3O+).
What is the equilibrium constant for water at 25 degrees Celsius, and how is it related to the concentrations of hydrogen and hydroxide ions?
-The equilibrium constant for water at 25 degrees Celsius is 10^-14. It is related to the concentrations of hydrogen and hydroxide ions in that their product at this temperature equals the equilibrium constant (H+ concentration * OH- concentration = 10^-14).
What is the pH of water at 25 degrees Celsius, and how is it calculated?
-The pH of water at 25 degrees Celsius is 7. It is calculated using the formula pH = -log[H+], where [H+] is the hydrogen ion concentration, which for pure water is 10^-7 M.
What is the pOH of water at 25 degrees Celsius, and how is it related to the pH?
-The pOH of water at 25 degrees Celsius is also 7. It is related to the pH through the equation pKw = pH + pOH, which equals 14 at 25 degrees Celsius for neutral water.
What happens to the pH and pOH values when a strong acid like hydrochloric acid is added to water?
-When a strong acid like hydrochloric acid is added to water, the pH decreases because the hydrogen ion concentration increases, while the pOH increases because the hydroxide ion concentration decreases due to the reaction with the added hydrogen ions.
What is the pH of a 1 M solution of hydrochloric acid?
-The pH of a 1 M solution of hydrochloric acid is 0, as the hydrogen ion concentration is equal to the molarity of the acid, which is 1 M, and pH = -log[H+] = -log(1) = 0.
What is the pOH of a 1 M solution of hydrochloric acid?
-The pOH of a 1 M solution of hydrochloric acid is 14, as the hydroxide ion concentration is significantly reduced due to the reaction with the excess hydrogen ions from the acid, leading to a pOH calculated by 14 - pH = 14 - 0 = 14.
How does the addition of a strong base like potassium hydroxide affect the pH and pOH of a solution?
-The addition of a strong base like potassium hydroxide increases the pH of the solution because it increases the hydroxide ion concentration, while decreasing the pOH since the hydrogen ion concentration is reduced due to the reaction with the added hydroxide ions.
What is the pH of a 1 M solution of potassium hydroxide?
-The pH of a 1 M solution of potassium hydroxide is 14, as the hydroxide ion concentration is equal to the molarity of the base, which is 1 M, and the pH is calculated by considering the reduced hydrogen ion concentration in the presence of excess hydroxide ions.
What is the pOH of a 1 M solution of potassium hydroxide?
-The pOH of a 1 M solution of potassium hydroxide is 0, as the pOH is calculated by 14 - pH = 14 - 14 = 0, given that the pH is 14 due to the high concentration of hydroxide ions.
Can the pH of a solution go below 0 or above 14 when dealing with very strong acids or bases?
-Yes, the pH of a solution can go below 0 or above 14 when dealing with very strong acids or bases. The scale is not absolute and is dependent on the hydrogen ion concentration in the case of acids or the reciprocal of the hydrogen ion concentration in the case of bases.
What is the significance of Le Chatelier's Principle in the context of adding acids or bases to water?
-Le Chatelier's Principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium moves to counteract the change. In the context of adding acids or bases to water, the principle explains how the system adjusts to the increase in hydrogen ions from acids or hydroxide ions from bases, shifting the water autoionization equilibrium to consume the excess ions and maintain the constant value of the equilibrium constant, pKw.
Outlines
🌊 Understanding Water's Autoionization and pH
This paragraph introduces the concept of water's autoionization, where water molecules can dissociate into hydrogen ions (protons) and hydroxide ions. It explains that at equilibrium, the concentration of hydrogen and hydroxide ions in pure water at 25°C is 10^-7 M, resulting in a pH of 7. The pKw value, which is the negative logarithm of the equilibrium constant for water autoionization, is established as 14. The relationship between pH, pOH, and pKw is also discussed, with the pH of water being equal to 7 and pOH being 7, making pKw 14 at 25°C.
🧪 Effect of Adding a Strong Acid to Water
This paragraph delves into the impact of introducing a strong acid, such as hydrochloric acid (HCl), to an aqueous solution. It explains that HCl fully dissociates in water, leading to a high concentration of hydrogen ions, which significantly lowers the pH to 0. The addition of HCl is akin to increasing the concentration of hydrogen ions by 10^7 times compared to pure water. The resulting decrease in hydroxide ion concentration is also discussed, with the pOH value being 14 due to the increased hydrogen ion concentration, as per the pKw constant of 14 at 25°C.
🌟 Exploring the Impact of a Strong Base on pH
This paragraph examines the effect of adding a strong base, such as potassium hydroxide (KOH), to water. It describes that KOH fully dissociates into potassium cations and hydroxide anions, leading to a high concentration of hydroxide ions and a resulting pH of 14. The paragraph highlights the inverse relationship between the presence of a strong base and the concentration of hydrogen ions, which significantly decreases. The pOH value for a strong base solution is 0, again referencing the pKw constant of 14 at 25°C.
Mindmap
Keywords
💡Autoionization
💡Equilibrium
💡Hydrogen Ion
💡pH
💡pOH
💡Hydrochloric Acid
💡Potassium Hydroxide
💡Le Chatelier's Principle
💡Temperature
💡pKw
💡Logarithm
Highlights
Water in equilibrium autoionizes into hydrogen ions and hydroxide ions, with hydronium being the form of hydrogen ions in water.
At 25 degrees Celsius, the equilibrium constant (Kw) for water is 10^-14, with hydrogen and hydroxide concentrations both at 10^-7.
The pKw, which is the negative log of the equilibrium constant of water, is 14 at 25 degrees Celsius.
pH and pOH are defined as the negative logs of the hydrogen ion and hydroxide ion concentrations, respectively.
In a neutral pH solution (pH 7), the concentrations of hydrogen ions and hydroxide ions are equal.
When hydrochloric acid (HCl) is added to water, it dissociates completely, increasing the hydrogen ion concentration.
A 1 Molar (1M) solution of hydrochloric acid in water results in a pH of 0 due to complete dissociation of HCl.
The pOH of a 1M HCl solution is 14, as per the pKw = pH + pOH rule at 25 degrees Celsius.
Potassium hydroxide (KOH) is a strong base that completely dissociates in water to form potassium cations and hydroxide anions.
A 1M solution of potassium hydroxide results in a pH of 14 and a pOH of 0, due to the high concentration of hydroxide ions neutralizing hydrogen ions.
The pH scale is not absolute; solutions can have pH values lower than 0 or higher than 14 with the addition of strong acids or bases.
Le Chatelier's Principle explains the shift in equilibrium due to the addition of acids or bases, where the system adjusts to counteract the change.
The addition of a strong acid increases the hydrogen ion concentration, causing a decrease in pH.
The addition of a strong base increases the hydroxide ion concentration, causing an increase in pH.
The relationship between pH and pOH is such that in a solution, increasing the concentration of one ion will decrease the concentration of the other.
The concept of pKw is crucial for understanding the behavior of water and its reactions with acids and bases.
The pH and pOH values are fundamental in determining the acidity or basicity of a solution.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: