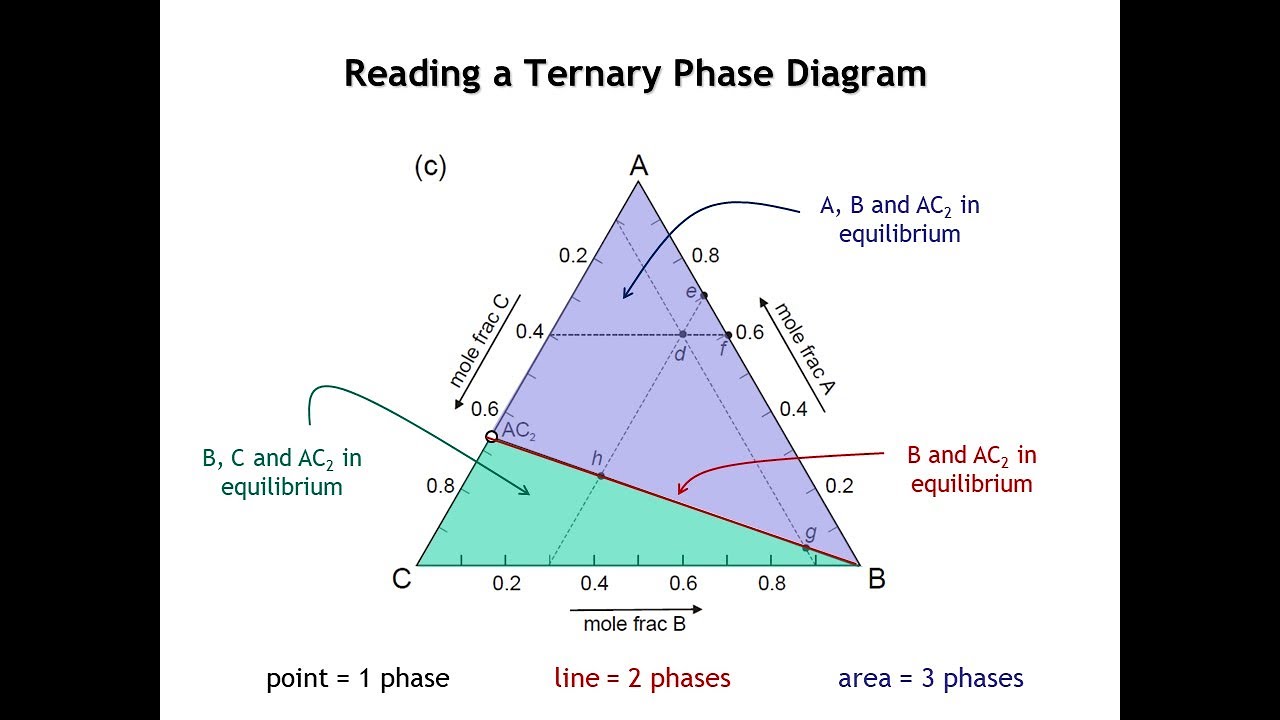
Phases present in the system
TLDRThis script discusses the use of phase diagrams to determine the phases present at a given point, focusing on the binary copper-nickel diagram. It explains how to read the diagram by identifying the phase regions and applying the 'one to one' rule, which states that between single-phase regions, a two-phase mixture must exist. The script uses examples of alloys with different compositions and temperatures to illustrate how to find the phases in equilibrium, highlighting the practicality of phase diagrams in material science.
Takeaways
- 📊 The script discusses the use of phase diagrams to answer specific questions about the phases present at given points, focusing on composition and temperature.
- 📌 The x-coordinate on the phase diagram represents composition, while the y-coordinate represents temperature, which are key to determining the phase(s) at equilibrium.
- 🔍 The binary copper-nickel phase diagram is used as an example to illustrate how to determine the phases present at a specific point.
- 🧪 In the copper-nickel diagram, different regions represent different phases: liquid, solid solution (alpha), and a two-phase region.
- 📍 Point A in the script is an example of a point in the alpha phase, with 60 weight percent nickel and equilibrated at 1200 degrees Celsius.
- 🔬 Point B is an example of a point in the two-phase region, with 50 weight percent nickel and equilibrated at 1250 degrees Celsius, indicating the presence of both alpha and liquid phases.
- 🔄 The script introduces the 'one to one rule' which states that in a binary phase diagram, moving horizontally from one single phase to another will always result in a two-phase region in between.
- 📈 The 'one to one rule' is a helpful guideline for understanding phase diagrams, especially when two-phase regions are not explicitly labeled.
- 🔑 Understanding phase diagrams is crucial for determining the phase(s) present in an alloy at a given temperature and composition.
- 📚 Phase diagrams are a fundamental tool in materials science, allowing for the prediction of phase behavior and the design of materials with desired properties.
- 🔍 The script emphasizes the importance of plotting the point of interest on the phase diagram to read the phases present, a straightforward method for interpreting phase diagrams.
Q & A
What is the primary purpose of a phase diagram?
-The primary purpose of a phase diagram is to determine the phases present at a given point, defined by composition and temperature, and to understand the equilibrium conditions of a material system.
How does the x-coordinate in a phase diagram represent composition in a binary alloy?
-In a phase diagram, the x-coordinate represents the composition of a binary alloy, typically in terms of weight percent of one of the components. The other component's proportion is implicitly understood as 100 minus the given component's percentage.
What does the y-coordinate in a phase diagram represent?
-The y-coordinate in a phase diagram represents temperature, which is a key parameter in determining the phase(s) present at equilibrium for a given composition.
What is the significance of the alpha phase in the context of the binary copper-nickel diagram?
-In the binary copper-nickel diagram, the alpha phase represents a solid solution phase where copper and nickel are mixed in the solid state, forming a single phase region.
How can you determine the phase(s) present at a specific point in a phase diagram?
-To determine the phase(s) present at a specific point, one must locate the point on the phase diagram according to its composition and temperature. The region in which the point falls indicates the phase(s) present at that condition.
What is the meaning of the 'one to one rule' in the context of binary phase diagrams?
-The 'one to one rule' states that in a binary phase diagram, if you move horizontally (along an isotherm) from one single phase region to another, there will always be a two-phase region in between, representing a mixture of the two single phases.
Why might two-phase regions sometimes not be labeled in some phase diagrams?
-Two-phase regions might not be labeled in some phase diagrams because they can always be inferred as the combination of the two single-phase regions that they border, making the one to one rule a helpful guideline for understanding phase diagrams.
What is a two-phase region and how does it differ from a single-phase region in a phase diagram?
-A two-phase region in a phase diagram is an area where two different phases coexist in equilibrium. This differs from a single-phase region, where only one phase is present at a given temperature and composition.
Can you provide an example of how to read the phase diagram for an alloy of 60 weight percent nickel at 1200 degrees Celsius?
-For an alloy of 60 weight percent nickel at 1200 degrees Celsius, you would plot this point on the phase diagram. If it falls within the alpha region, the alloy is in the alpha phase. If it falls in the two-phase region, the alloy is a mixture of two phases, such as alpha and liquid.
What is the composition of an alloy that is 50 weight percent nickel and 50 weight percent copper?
-An alloy that is 50 weight percent nickel is also 50 weight percent copper by default, as these percentages add up to 100%. This is a 50-50 alloy in terms of composition.
How does the phase diagram help in understanding the behavior of an alloy at different temperatures?
-A phase diagram helps in understanding the behavior of an alloy at different temperatures by showing the regions where different phases are stable. By looking at the temperature on the y-axis and the composition on the x-axis, one can determine the phase(s) present and how they change with temperature.
Outlines
📊 Understanding Phase Equilibrium in Binary Alloys
This paragraph introduces the fundamental use of phase diagrams to determine the phases present at a specific point defined by composition and temperature. The binary copper-nickel phase diagram is used as an example to illustrate how to identify the phase(s) in equilibrium at a given point. The explanation covers how to interpret the x (composition) and y (temperature) coordinates on the diagram and how to identify single-phase regions (like alpha phase) and two-phase regions. The paragraph also provides examples of how to determine the phase(s) for alloys with different compositions at specific temperatures, such as an alloy with 60 weight percent nickel at 1200 degrees Celsius being in the alpha phase, and an alloy with 50 weight percent nickel at 1250 degrees Celsius being in a two-phase region with both alpha and liquid phases present.
📐 The One-to-One Rule in Phase Diagrams
The second paragraph delves into the concept of the one-to-one rule in binary phase diagrams, which is a principle that helps predict the presence of two-phase regions based on single-phase regions. It explains that when moving horizontally across a phase diagram (along an isotherm) from one single phase to another, there will always be an intermediate region where a mixture of these two phases exists. The paragraph uses the liquid and alpha phases as an example to demonstrate this rule, noting that if a phase diagram only shows single-phase regions, the two-phase regions can be inferred as the combination of those single phases. The one-to-one rule is highlighted as a useful tool for interpreting phase diagrams, especially when two-phase regions are not explicitly labeled.
Mindmap
Keywords
💡Phase Diagram
💡Phases
💡Equilibrium
💡Binary Alloy
💡Composition
💡Temperature
💡Solid Solution Phase
💡Two-Phase Region
💡Isotherm
💡One to One Rule
Highlights
Introduction of phase diagrams to answer three fundamental questions about phases at a given point in a binary system.
Explanation of how the x coordinate represents composition and the y coordinate represents temperature in a phase diagram.
Direct method to determine the phases in equilibrium at a given composition and temperature by examining the phase diagram.
Use of the binary copper-nickel diagram as a familiar example to illustrate phase determination.
Identification of the liquid phase, solid solution phase (alpha), and two-phase region in the copper-nickel diagram.
Demonstration of how to determine the phase at a specific point (e.g., point A) by its composition and temperature.
Clarification of the convention for binary alloys where one component's proportion defines the composition.
Example of an alloy with 60 weight percent nickel equilibrated at 1200 degrees Celsius, identifying the alpha phase.
Introduction of another alloy (alloy B) with a composition of 50 weight percent nickel at 1250 degrees Celsius.
Explanation of the presence of two phases (alpha and liquid) in the two-phase region of the phase diagram.
Illustration of the one-to-one rule in binary phase diagrams, where a transition from one phase to another indicates a two-phase region.
Rule explanation that moving horizontally across a phase boundary in a binary diagram always results in a two-phase region.
Importance of the one-to-one rule for understanding phase diagrams and the presence of two-phase regions.
Note on the common practice of phase diagrams in literature, which may only list single-phase regions due to the predictable nature of two-phase regions.
Advantage of the one-to-one rule in interpreting phase diagrams, especially when two-phase regions are not explicitly labeled.
Transcripts
Browse More Related Video
5.0 / 5 (0 votes)
Thanks for rating: