States of matter | States of matter and intermolecular forces | Chemistry | Khan Academy
TLDRThis script delves into the fundamental states of matter, focusing on the transition from solid to liquid to gas, using water as a primary example. It explains how temperature affects molecular motion and interactions, leading to phase changes. The video illustrates the concept of polar bonds and how they influence the structure of solids, the sliding motion in liquids, and the independent movement in gases. It further explores the energy exchanges during phase transitions, introducing terms like 'heat of fusion' and 'heat of vaporization,' and clarifies the distinction between temperature changes and energy used for changing states of matter.
Takeaways
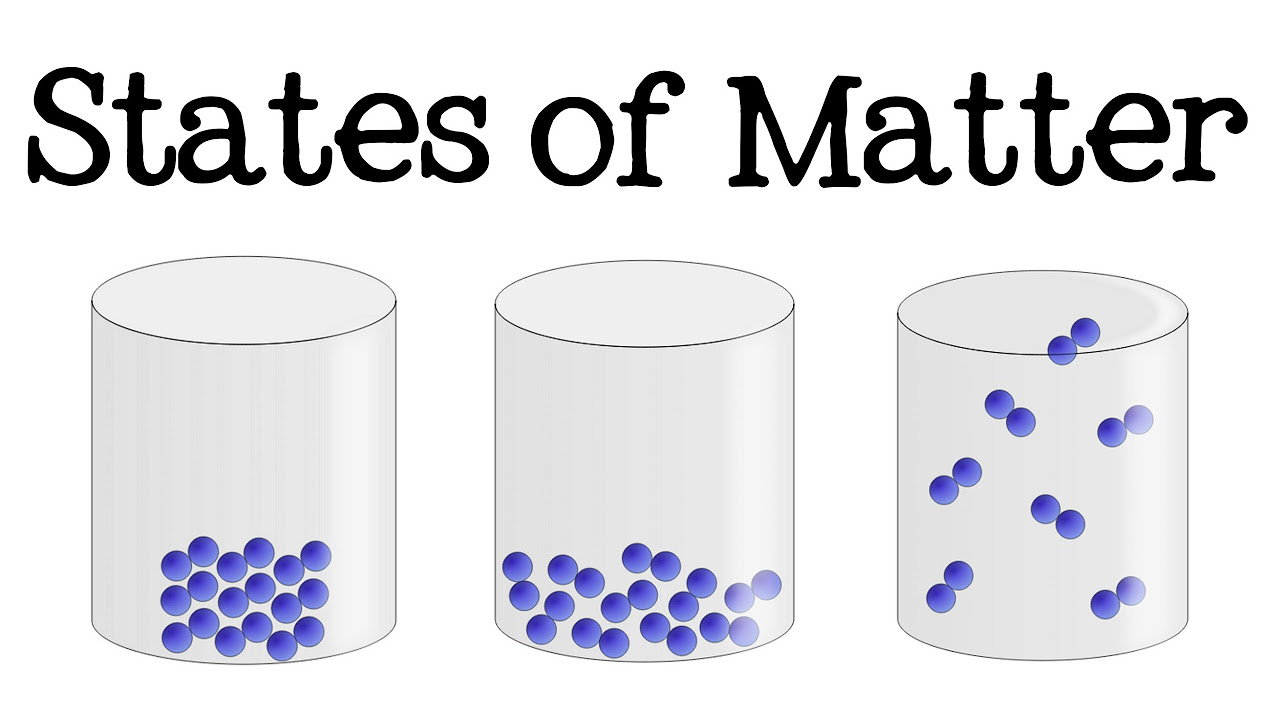
- 🌡️ The three common states of matter are solid, liquid, and gas, with a fourth state possible at very high temperatures.
- 🧊 Water serves as a primary example to illustrate the transition between states, with ice representing the solid state, liquid water the liquid state, and water vapor or steam the gaseous state.
- 🔬 The polarity of water molecules, due to the electronegativity difference between hydrogen and oxygen, results in partial charges that influence intermolecular interactions.
- 🔗 In the solid state, low kinetic energy leads to the formation of a lattice structure where molecules are held together by polar bonds.
- 🔥 As heat is added to a solid, the molecules vibrate more but maintain their fixed positions, increasing the average kinetic energy or temperature.
- 💧 The transition from solid to liquid involves overcoming the polar bonds with enough kinetic energy, allowing molecules to slide past each other without completely separating.
- 🌡️ The phase change from solid to liquid occurs at a constant temperature, where the added heat does not increase temperature but changes the state by breaking intermolecular bonds.
- 🌫️ In the liquid state, molecules have enough kinetic energy to move past each other but remain attracted, unlike in the gaseous state where molecules move independently.
- 🌡️ The phase change from liquid to gas also occurs at a constant temperature, requiring a significant amount of heat to vaporize the liquid into gas without a temperature rise.
- 🔑 The terms 'heat of fusion' and 'heat of vaporization' refer to the specific amounts of heat required to change matter from solid to liquid and from liquid to gas, respectively.
- 📊 A phase change diagram illustrates the relationship between heat added and temperature changes, highlighting the constant temperature periods during phase transitions.
Q & A
What are the three common states of matter?
-The three common states of matter are solid, liquid, and gas.
What is a special characteristic of water that relates to its states of matter?
-Water is unique in that its solid state (ice) is less dense than its liquid state, which is why ice floats on water.
What is the role of electronegativity in the formation of water molecules?
-Electronegativity causes the electrons in the bonds between hydrogen and oxygen in water molecules to spend more time around the oxygen, resulting in a partial negative charge on the oxygen and a partial positive charge on the hydrogen.
How does the kinetic energy of molecules relate to the state of matter?
-The kinetic energy of molecules determines their state of matter. Low kinetic energy results in a solid state, moderate kinetic energy leads to a liquid state, and high kinetic energy causes a gaseous state.
What causes the molecules in a solid to maintain a fixed structure?
-In a solid, the molecules have low kinetic energy, leading to strong intermolecular forces that keep them in a fixed, lattice-like structure.
What happens to the molecules in a solid when heat is added?
-When heat is added to a solid, the molecules gain kinetic energy and start to vibrate more, but they maintain their fixed structure.
What is the term for the bonds formed between polar water molecules due to their partial charges?
-The bonds formed between polar water molecules are called polar bonds, due to the partial positive and negative charges on the molecules.
What is the phase change diagram and what does it represent?
-A phase change diagram is a graphical representation that shows the relationship between the amount of heat added to a substance and its temperature, illustrating the states of matter and phase transitions.
What is the term used to describe the amount of heat required to change a substance from a solid to a liquid?
-The term used to describe the amount of heat required to change a substance from a solid to a liquid is the heat of fusion.
Why does the temperature of a substance remain constant during a phase change?
-The temperature remains constant during a phase change because the added heat is used to break the intermolecular bonds and change the state of the substance, rather than increasing its kinetic energy or temperature.
What is the significance of the terms 'enthalpy' and 'heat of vaporization' in the context of phase changes?
-Enthalpy refers to the heat content of a system and is closely related to the heat involved in phase changes. The heat of vaporization is the amount of heat required to change a substance from a liquid to a gas.
Outlines
🧊 Understanding States of Matter and Water's Behavior
This paragraph introduces the three common states of matter—solid, liquid, and gas—and uses water as a primary example to illustrate the transitions between these states. It explains how temperature affects these states, with ice representing the solid state, liquid water as the liquid state, and water vapor or steam as the gaseous state. The discussion also delves into the molecular structure of water, highlighting the polar nature of the water molecule due to the electronegativity of oxygen, which leads to the formation of hydrogen bonds between molecules. These bonds are crucial in establishing the solid structure at low kinetic energy and how increased energy leads to the breaking of this structure, transitioning water from solid to liquid to gas.
🔥 Transition from Solid to Liquid and Liquid to Gas
The second paragraph explores the process of phase transitions, specifically focusing on how heat affects the movement of molecules within a solid and leads to the formation of a liquid state. It explains that as kinetic energy increases due to added heat, the polar bonds between molecules weaken, allowing them to slide past each other, which characterizes the liquid state. Further addition of heat results in the complete separation of molecules, leading to the gaseous state where molecules move independently with high kinetic energy. The paragraph also touches on the visibility of gases due to the greater distance between molecules, allowing more light to pass through, and sets the stage for discussing how to measure the heat involved in these phase changes.
📊 Phase Change Diagram and Enthalpy
This paragraph introduces the concept of a phase change diagram, which is used to represent the relationship between heat added to a substance and the resulting temperature changes. It explains the terms 'enthalpy' and 'change in enthalpy,' equating them to heat content and changes in heat, respectively. The paragraph clarifies that these terms, often used in chemistry, are essentially different ways of discussing heat. The discussion includes the representation of solid, liquid, and gas phases on the diagram, with specific focus on the temperatures at which water freezes, melts, boils, and vaporizes. It emphasizes the constant temperature periods during phase transitions, where heat is added without a rise in temperature, indicating a change in state.
🔄 Heat's Role in Phase Transitions and Potential Energy
The final paragraph delves deeper into the role of heat during phase transitions, particularly the heat of fusion and heat of vaporization. It explains that during these transitions, heat is not used to increase the temperature but rather to break molecular bonds, effectively increasing the potential energy of the system. The paragraph uses the analogy of a person in an airplane to illustrate the concept of potential energy and relates it back to the molecular level, where the energy is used to move molecules apart during phase changes. It also discusses the concept of zero-degree water and 100-degree water, explaining that these states do not necessarily imply solid or gas forms, respectively, and that additional energy is required to induce these phase changes completely.
Mindmap
Keywords
💡States of Matter
💡Electronegativity
💡Polar Bonds
💡Lattice Structure
💡Kinetic Energy
💡Phase Change
💡Heat of Fusion
💡Heat of Vaporization
💡Potential Energy
💡Enthalpy
💡Phase Change Diagram
Highlights
The three common states of matter are solid, liquid, and gas, with a fourth state possible at very high temperatures.
Water serves as a primary example to illustrate the transition between states of matter.
Solids form at colder temperatures, liquids at warmer temperatures, and gases at even higher temperatures.
The polarity of water molecules, with oxygen being more electronegative, leads to partial charges and hydrogen bonding.
In the solid state, molecules have low kinetic energy, forming a stable lattice structure due to polar bonds.
As heat is added to a solid, molecules vibrate more but maintain their structure.
A certain amount of heat is required to break the bonds between molecules, transitioning from solid to liquid.
In the liquid state, molecules have enough kinetic energy to slide past each other but remain attracted.
Further addition of heat can lead to complete separation of molecules, transitioning to a gaseous state.
Gaseous molecules have high kinetic energy and do not touch each other, moving independently.
The density of states of matter varies, with gases generally having lower density than liquids and solids.
The phase change diagram illustrates the relationship between heat added and temperature changes.
Heat of fusion is the energy required to change a substance from solid to liquid without a temperature increase.
Heat of vaporization is the energy needed to transition from liquid to gas, also without an immediate temperature rise.
Enthalpy, often used in chemistry, is essentially the heat content or change in heat within a system.
Potential energy increases during phase changes as molecules are moved apart against natural attractive forces.
The temperature at which water boils (100 degrees Celsius) is a point of interest where heat is added without an immediate temperature increase.
Water at zero degrees Celsius is not necessarily ice, and water at 100 degrees Celsius is not necessarily steam, as additional energy is required for phase changes.
The concept of potential energy and work is introduced to explain the energy changes during phase transitions.
Transcripts
Browse More Related Video

Phase Changes

Phase Changes, Heats of Fusion and Vaporization, and Phase Diagrams

Phase Changes | Chemistry | The Good and the Beautiful

Phases of Matter and Phase Change Diagrams

Latent Heat of Fusion and Vaporization, Specific Heat Capacity & Calorimetry - Physics
3 States of Matter for Kids (Solid, Liquid, Gas): Science for Children - FreeSchool
5.0 / 5 (0 votes)
Thanks for rating: