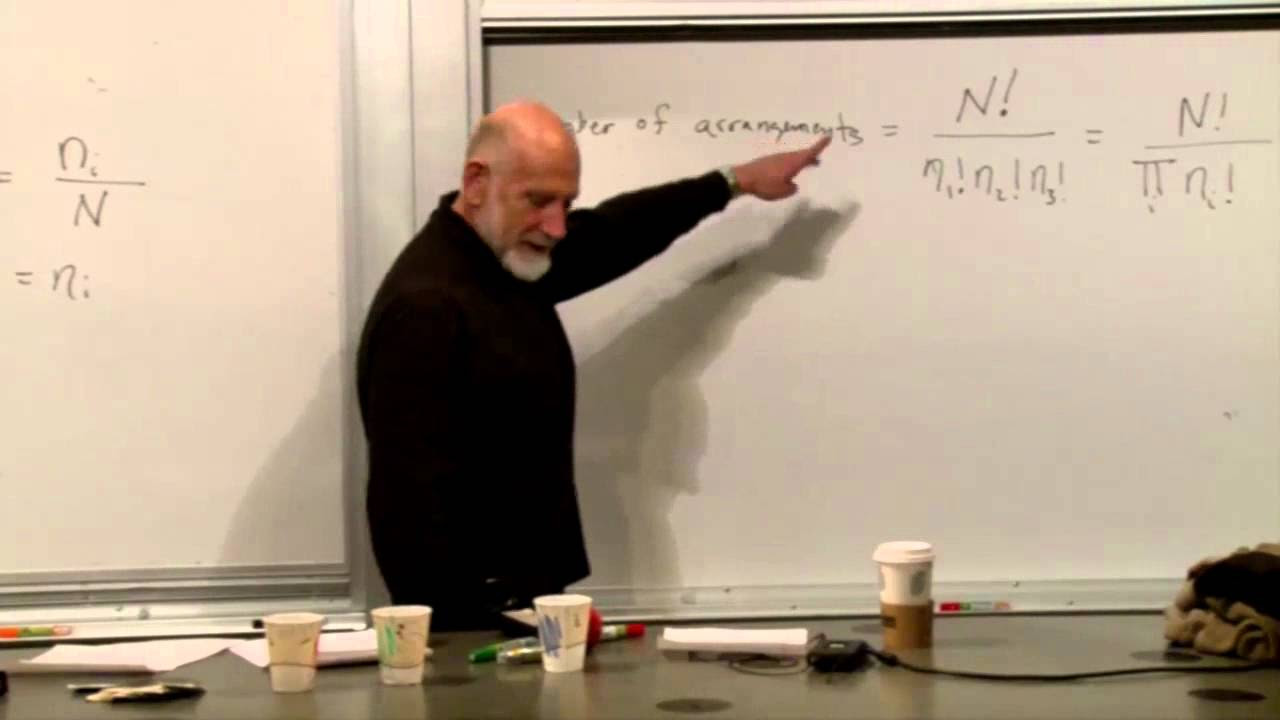Wayne Myrvold: The Maxwellian view of thermodynamics & statistical mechanics
TLDRIn this philosophical exploration, Wayne Merville delves into the Maxwellian view of statistical mechanics, positing thermodynamics as a resource theory. He discusses the historical evolution of the second law of thermodynamics, the role of probabilities in statistical mechanics, and the implications of Maxwell's demon. Merville emphasizes the importance of understanding thermodynamics not just as a physical law, but as a framework for how agents with limited information can perform tasks, suggesting a shift from viewing entropy as an inherent property to a measure relative to an agent's capabilities.
Takeaways
- 📚 The talk discusses the Maxwellian view of statistical mechanics, emphasizing thermodynamics as a resource theory and the role of information in physical systems.
- 🌐 The development of resource theories, especially quantum resource theories, has been influenced by the idea that physical properties like entanglement can be used for practical tasks.
- 🔬 Maxwell's early formulation of what became the second law of thermodynamics introduced the concept of heat transfer requiring additional effects, challenging the idea of spontaneous heat flow.
- 🔄 The kinetic theory of gases and the realization that random molecular motion could lead to apparent violations of the second law led to the incorporation of probabilities in statistical mechanics.
- 🧪 James Clerk Maxwell's thought experiment, known as Maxwell's demon, proposed a scenario where an agent could use information about molecular velocities to violate the second law of thermodynamics.
- 💡 The idea that thermodynamics is a statistical law, true on average for large populations but allowing for small fluctuations, was a significant shift in understanding the second law.
- 📈 Maxwell's view on thermodynamics as a science of energy interchangeability between heat and work, and how this distinction vanishes at a fundamental level with complete information about a system.
- 📚 The historical context shows that the concept of thermodynamics as a resource theory is not new, but has been present since Maxwell's time, highlighting the use of non-equilibrium as a resource.
- 🤔 The script raises questions about the subjective nature of entropy and the role of an observer's ability to manipulate and measure physical systems in defining thermodynamic properties.
- 🔮 The potential for future advances in manipulating individual molecules suggests that thermodynamic concepts may need to be extended or modified for systems at the quantum level.
- 📝 The importance of recognizing the epistemic and physical aspects of thermodynamics, including the role of probability and the limitations of our knowledge and control over physical systems.
Q & A
What is the main theme of the conference where Wayne Merville is giving the talk?
-The main theme of the conference is 'thermodynamics as a resource theory,' which explores the concept of thermodynamics within a framework of resource theories that deal with the use of physical properties to accomplish tasks.
What is the significance of the Maxwellian view in the context of statistical mechanics?
-The Maxwellian view is significant because it historically framed thermodynamics as a way to use non-equilibrium as a resource for performing useful tasks, such as work, and it introduced the idea of statistical mechanics, which incorporates probabilities due to the random motion of molecules.
What is the historical context of the second law of thermodynamics as discussed by Wayne Merville?
-The historical context involves the early formulations of the second law by Kelvin and Clausius, the kinetic theory of gases, and the realization by Maxwell and others that the law could not be strictly true due to the probabilistic nature of molecular motion, leading to the development of statistical mechanics.
What is the concept of 'Maxwell's demon' and how does it relate to the second law of thermodynamics?
-Maxwell's demon is a thought experiment involving an agent that could, in theory, separate faster and slower molecules in a gas by using a partition, thus seemingly violating the second law of thermodynamics. It highlights the role of information and manipulation at the microscopic level in understanding the second law.
How did the understanding of the second law of thermodynamics evolve over time?
-Initially, the second law was considered a strict impossibility, but over time, scientists like Maxwell, Boltzmann, and others realized that it is more about probabilities and expectations, acknowledging that fluctuations can occur but are not reliable for performing work.
What is the role of probability in the development of statistical mechanics?
-Probability plays a central role in statistical mechanics as it allows for the understanding of the behavior of large numbers of particles, the approach to equilibrium, and the concept that deviations from expected behavior, while dynamically possible, are statistically improbable.
Why did Maxwell suggest that thermodynamics could be seen as a resource theory?
-Maxwell suggested this perspective because he recognized that thermodynamics could be used to perform tasks, such as work, by utilizing non-equilibrium states as a resource, which aligns with the modern concept of resource theories in physics.
What is the distinction Maxwell made between 'available energy' and 'dissipated energy'?
-Available energy is energy that can be directly used for a desired purpose, while dissipated energy is energy that cannot be harnessed or directed due to its form, such as the random motion of molecules in heat.
How does the concept of thermodynamics as a resource theory relate to the idea of information in physics?
-Thermodynamics as a resource theory incorporates the idea that information about the physical properties of a system can be used as a resource to accomplish tasks, highlighting the role of knowledge and manipulation in the physical world.
What is the philosophical implication of viewing thermodynamics as a resource theory?
-The philosophical implication is that it shifts the focus from seeing thermodynamics as solely about the physical world to recognizing it as a framework for understanding how agents with limited knowledge and manipulation abilities can use physical properties to perform tasks.
Outlines
📚 Introduction to Thermodynamics as a Resource Theory
The speaker, Wayne Merville, introduces the concept of thermodynamics as a resource theory, a framework for understanding how physical properties can be utilized to perform tasks. He emphasizes the growing interest in this field, with developments like quantum resource theories, which have transformed our understanding of properties like entanglement. Merville critiques the traditional philosophical focus on fundamental properties, advocating for the importance of resource theories in both quantum information and thermodynamics.
🔄 Historical Perspective on Thermodynamics and Maxwell's View
Merville provides a historical context for thermodynamics, highlighting Maxwell's early insights that non-equilibrium could be used as a resource. He discusses Maxwell's formulation of what would later be known as the second law of thermodynamics and the subsequent development of statistical mechanics. The speaker also touches on the famous 'Maxwell's demon' thought experiment, which challenges the strict interpretation of the second law by suggesting that an intelligent agent could reverse entropy increases.
🧩 Maxwell's Demon and the Concept of Irreversibility
The talk delves into the implications of Maxwell's demon, which questions the irreversible nature of thermodynamic processes. Merville explains how the demon could, in theory, use information about molecular velocities to separate faster molecules from slower ones, thereby decreasing entropy. He also discusses the annotations on Maxwell's letter, which introduced the idea of velocity reversal as a means to challenge the second law of thermodynamics.
🔄 The Evolution of Understanding the Second Law of Thermodynamics
Merville explores how the understanding of the second law of thermodynamics evolved from a strict impossibility to a statistical probability. He mentions the contributions of various scientists, including Maxwell, who recognized that the second law is not an absolute but rather a statement of high improbability. The speaker also discusses the shift in perspective that occurred in the 1870s, moving away from the original strict formulations of the second law.
🔄 Maxwell's Insights on the Statistical Nature of the Second Law
The speaker highlights Maxwell's recognition of the statistical nature of the second law of thermodynamics. Maxwell proposed that the law holds true on average for a large population of molecules but allows for fluctuations that could, in principle, lead to violations of the law in small systems. Merville discusses Maxwell's view that thermodynamics is a statistical law that emerges from the behavior of large numbers of molecules.
🔄 Thermodynamics, Work, and the Concept of Dissipated Energy
Merville discusses Maxwell's perspective on the distinction between work and heat in thermodynamics. He explains that Maxwell viewed this distinction as a matter of our limited ability to manipulate and perceive individual molecular motions. The speaker also touches on the concept of dissipated energy, which Maxwell considered to be relative to an observer's capacity to utilize energy.
🔄 The Role of Information and Manipulation in Thermodynamics
The talk explores the role of information and manipulation in thermodynamics, as seen through Maxwell's work. Merville suggests that thermodynamics is fundamentally about how beings with limited knowledge and manipulation abilities can use the information they have to perform useful work. He also discusses the idea that thermodynamics is about the interchangeability of heat and work and how Maxwell's views on this were quite revolutionary.
🔄 Maxwell's View on the Subjectivity of Entropy and Energy
Merville delves into Maxwell's view that entropy and the concept of dissipated energy are relative to the observer's ability to manipulate and perceive the system. He explains that Maxwell considered these concepts to be means-relative, depending on the capabilities of the observer. The speaker also discusses the implications of this view for the understanding of thermodynamics and the role of subjective elements in physical laws.
🔄 Reflections on Maxwell's Contributions to Statistical Mechanics
The speaker reflects on the broader implications of Maxwell's work on statistical mechanics and thermodynamics. Merville emphasizes the importance of Maxwell's insights on the role of information, manipulation, and subjectivity in physical laws. He also discusses the potential for Maxwell's ideas to inform current debates in quantum thermodynamics and the role of epistemic considerations in our understanding of physical systems.
🔄 Conclusion and Speculations on the Future of Thermodynamics
In conclusion, Merville speculates on the future of thermodynamics and the potential for advancements in our understanding of physical systems. He suggests that Maxwell's work may have implications for quantum thermodynamics and the role of information processing in physical laws. The speaker also encourages a reevaluation of Maxwell's contributions and a deeper exploration of the philosophical implications of his ideas.
Mindmap
Keywords
💡Thermodynamics
💡Statistical Mechanics
💡Maxwell's Demon
💡Resource Theory
💡Second Law of Thermodynamics
💡Quantum Resource Theory
💡Entanglement
💡Kinetic Theory of Gases
💡H-Theorem
💡Time Reversal Invariance
💡Neo-Boltzmannian View
Highlights
Introduction of the Maxwellian view of statistical mechanics within the context of thermodynamics as a resource theory.
The development of a general framework for resource theories and their application in physical systems to accomplish tasks.
The significance of quantum resource theories in revolutionizing the understanding of properties like entanglement for practical tasks.
Historical perspective on thermodynamics as a theory of using non-equilibrium as a resource, traced back to Maxwell's original thoughts.
James Clerk Maxwell's early formulation of what would later be known as the second law of thermodynamics in 1854.
The kinetic theory of gases and the realization that probabilities must be considered in thermodynamics, leading to statistical mechanics.
The famous 'H-theorem' of Boltzmann and the controversy surrounding the derivation of entropy from mechanics of collisions.
Maxwell's letter to Peter Guthrie Tait discussing the possibility of violating the second law of thermodynamics with precise manipulation.
The concept of Maxwell's demon as an agent capable of separating molecules based on their velocities, challenging the second law of thermodynamics.
The role of probabilities and the idea that approaching equilibrium is probable but not certain due to dynamic possibilities.
Maxwell's view that thermodynamics is a statistical law true on average for large populations, not a strict impossibility.
The distinction between work and heat in thermodynamics and Maxwell's perspective on their interconvertibility.
Maxwell's idea that thermodynamics is about how beings with limited knowledge and manipulation abilities can use resources to perform work.
The philosophical implications of thermodynamics as a resource theory and its potential impact on the understanding of physical properties.
The historical shift from viewing the second law of thermodynamics as an impossibility to a statistical truth.
The importance of recognizing the role of agents and their abilities in the physical theories, challenging the traditional view of physics.
The potential for future advances in thermodynamics and statistical mechanics that consider the manipulation of individual molecules.
The relevance of Maxwell's work to current discussions on quantum thermodynamics and the role of information in physical processes.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: