Mass balance and Significant Figures
TLDRThis video script offers a detailed guide on using mass balances for accurate weighing in scientific experiments. It outlines a step-by-step method for weighing an empty container, transferring a sample, and determining the sample's mass with precision. The video also addresses the concept of significant figures, explaining their importance and how to apply them in calculations to ensure the reliability of experimental results. The script emphasizes the need for accuracy and consistency in scientific measurements and calculations.
Takeaways
- 🔍 Use a mass balance to weigh known quantities of a sample for experiments requiring quantitative mass results.
- 📐 Begin with accurately weighing the empty weighing bottle in an accurate balance room, ensuring the balance is level and the environment is stable.
- ⚖️ Press the 'tare' or '0' button to zero the scale before placing the weighing bottle on the balance.
- 📝 Record the mass of the container and note the balance number, maintaining the same balance throughout the experiment.
- 🔄 Use rough balances to roughly transfer approximately one gram of the sample to the weighing bottle.
- 🧑🔬 Ensure the balance pan is clean before placing the weighing bottle on it and zero the balance with the bottle on the pan.
- 🚫 Avoid transferring the sample directly onto the balance pan; do this off the balance.
- 📉 Add sample to the weighing bottle until the mass is near one gram, accepting slight deviations like 0.96 grams or 1.05 grams.
- 🔄 Return to the accurate balance room to zero the balance and weigh the sample in the bottle, recording the mass to all decimal places shown.
- ➖ Subtract the mass of the empty weighing bottle from the mass of the bottle with contents to find the mass of the added sample.
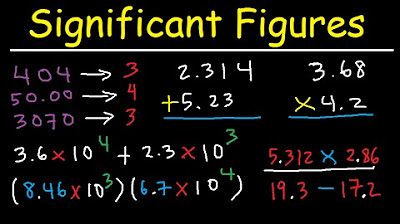
- 🔢 Understand the concept of significant figures, which is crucial for accurately reporting scientific measurements and maintaining the reliability of results.
- 📚 Follow the rules for significant figures, such as knowing which zeros are significant and how to handle them in calculations.
- 🧮 Be mindful of the number of decimal places and significant figures when performing calculations, rounding off to the appropriate number of significant figures.
- 📘 Exact numbers like the number of days in a week or atoms in a molecule do not have uncertainty and should not affect the count of significant figures.
Q & A
What are mass balances used for in a laboratory setting?
-Mass balances are used to weigh known quantities of a sample accurately, which is essential for experiments requiring quantitative mass results.
Why is it important to use a mass balance correctly?
-Using a mass balance correctly ensures accurate mass measurements, which are crucial for the reliability of experimental results.
What is the first step in using a mass balance as per the video transcript?
-The first step is to accurately weigh the empty weighing bottle in the accurate balance room, ensuring the balance is level and glass windows are properly closed.
What should you do if the mass balance is not level?
-If the mass balance is not level, you should ask your demonstrator or lab staff for assistance with leveling the mass balance.
How do you ensure the balance is zeroed before weighing?
-Press the 'tare' or '0' button to zero the scale before placing the weighing bottle on the balance.
What is the purpose of noting the balance number used during the experiment?
-Noting the balance number ensures that the same balance is used throughout the experiment, maintaining consistency in measurements.
How do you perform a rough transfer of the sample to the weighing bottle?
-Use the rough balances in the rough balance room, ensuring the balance pan is clean, and transfer some of the sample into the weighing bottle without placing it directly on the balance pan.
What is the acceptable range for the rough mass measurement of the sample?
-The rough mass measurement can be slightly above or below one gram, for example, 0.96 grams or 1.05 grams are perfectly acceptable.
Why is it necessary to record the mass of the weighing bottle and its contents to all decimal places shown on the balance?
-Recording to all decimal places ensures accuracy and accounts for any zeros that may be significant in the measurement.
How do you determine the mass of the sample added to the weighing bottle?
-Subtract the mass of the empty weighing bottle from the mass of the weighing bottle with its contents to determine the mass of the sample added.
What is the concept of significant figures and why is it important in scientific measurements?
-Significant figures are a measure of the precision of a number, indicating the reliability of scientific measurements. They ensure that results are reported with the correct level of certainty.
How can you identify the number of significant figures in a given value?
-Nonzero digits are always significant, zeros between nonzero digits are significant, and zeros before a nonzero number are not significant. For example, 0.085 has two significant figures, while 101 has three.
What are the rules to follow when performing calculations using experimentally determined values?
-When performing calculations, consider the number of decimal places in the values used, report the final answer to the lowest number of significant figures seen in the values, and do not blindly copy numbers from your calculator.
How should you handle exact numbers when considering significant figures in calculations?
-Exact numbers, such as the number of days in a week or oxygen atoms in a water molecule, do not have uncertainty and should not be included when considering significant figures.
Outlines
🔍 Accurate Mass Weighing and Balance Usage
This paragraph explains the importance of mass balances in experiments requiring quantitative mass results. It provides a step-by-step guide on how to accurately weigh an empty weighing bottle, use rough balances to estimate sample mass, and then accurately weigh the sample in the weighing bottle. The process involves zeroing the scale, transferring the sample without direct contact with the balance, and recording the mass to the correct decimal places. The paragraph also introduces the concept of significant figures, emphasizing their importance in maintaining the reliability of experimental results.
📚 Significant Figures and Calculation Precision
The second paragraph delves into the concept of significant figures, a topic that can be challenging for students. It outlines the rules for determining the number of significant figures in a value, such as considering nonzero digits as significant and zeros between nonzero digits. The paragraph also addresses how to handle significant figures in scientific notation and provides guidance on performing calculations with experimental values. It stresses the importance of reporting answers with the correct number of significant figures and not relying solely on calculator results, to ensure the accuracy and reliability of scientific measurements and calculations.
Mindmap
Keywords
💡Mass Balances
💡Significant Figures
💡Weighing Bottle
💡Rough Balance
💡Accurate Balance
💡Zeroing the Scale
💡Sample Residue
💡Nonzero Digits
💡Decimal Places
💡Molar Mass
💡Exact Numbers
Highlights
Mass balances are essential for experiments requiring quantitative mass results.
A step-by-step method is provided for accurately weighing samples.
Ensure the balance is level and zeroed before weighing.
Weigh the empty weighing bottle first in an accurate balance room.
Use the same balance throughout the experiment for consistency.
Transfer a rough estimate of the sample using rough balances.
Clean the balance pan before each use.
Do not transfer samples directly onto the balance pan.
Adjust the sample mass in the weighing bottle until near one gram.
Record the mass of the weighing bottle and its contents to the nearest decimal.
Subtract the mass of the empty bottle to find the sample's mass.
For quantitative transfers, weigh the emptied bottle to determine exact sample transfer.
Significant figures are crucial for accurate scientific measurements.
Four significant figures imply the first three are certain, the last has uncertainty.
Rules for identifying significant figures include the significance of zeros.
Different ways to express numbers impact the perceived accuracy.
Calculations should consider the number of decimal places and significant figures.
Report final answers to the least number of significant figures used in calculations.
Exact numbers, like constants, do not affect the count of significant figures.
The video covers the correct use of mass balances and significant figures for reliable measurements.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: