Sample Size Calculation Made Easy - Case Control Study Design -HeDaL
TLDRThe video script is an educational session on the design and sample size calculation for case-control studies. It begins with a discussion on the fundamental difference between case-control and cohort studies, emphasizing the importance of understanding the timing of exposure and outcome. The session then delves into the factors influencing sample size calculation, such as the odds ratio, the distribution of the risk factor in the population, and the desired statistical power and significance level. Practical examples are used to illustrate how these factors impact the required sample size. The presenter also addresses common challenges in case-control studies, such as the selection of appropriate controls and the management of confounding factors. The session concludes with a Q&A segment where participants' questions about the application of case-control study methodologies are answered.
Takeaways
- 📚 The session focused on calculating sample size for case-control studies, contrasting with previous discussions on cross-sectional studies.
- 🔍 Case-control studies were differentiated from cohort and cross-sectional studies, emphasizing their unique methodology and application.
- 📉 The importance of understanding exposure and outcome in study design was highlighted, with examples provided to illustrate the concepts.
- 🤔 A hypothesis about neonatal jaundice affecting IQ in childhood was used as a running example to engage participants in the discussion.
- 👶 The session clarified that starting with exposure and measuring outcome later defines a cohort study, not a case-control study.
- 🧐 The necessity of a good pre-existing database for case-control studies was underscored, noting the challenges of incomplete records.
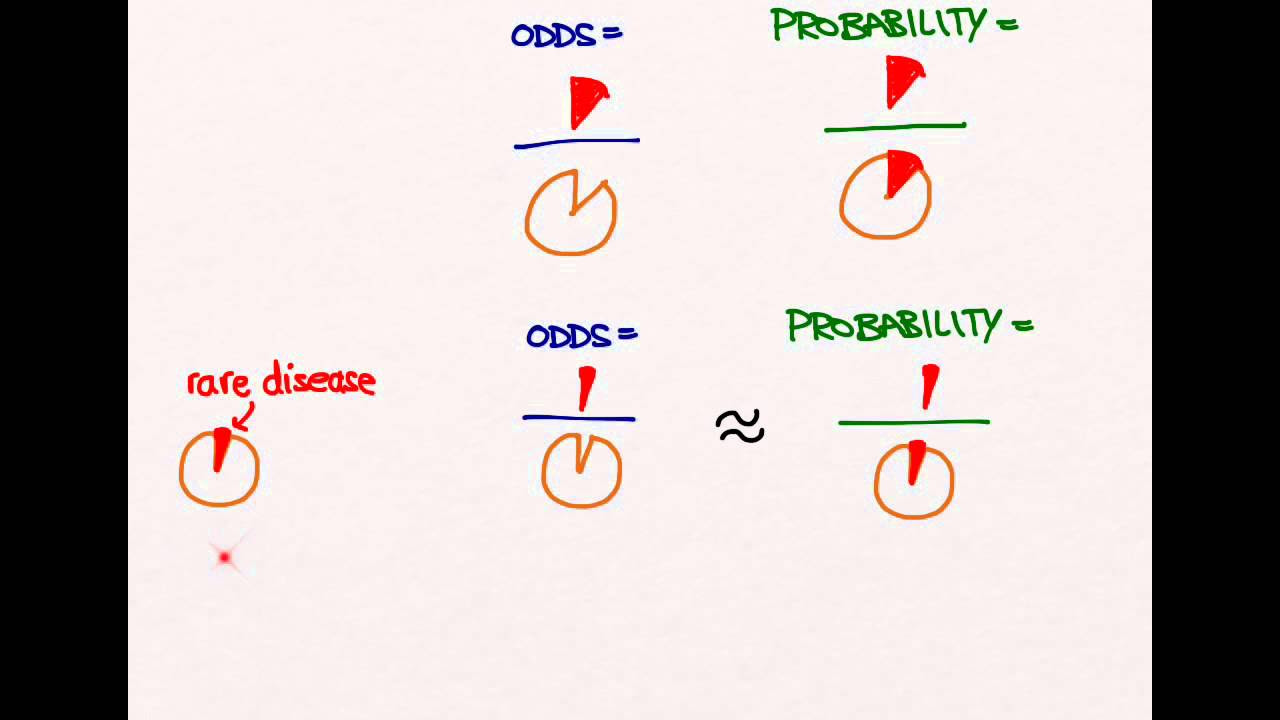
- 📝 The measure of association in case-control studies is the odds ratio, which was explained in detail with examples.
- ⚖️ The difference between odds ratio and risk ratio was clarified, noting that odds ratios remain consistent regardless of control group size, unlike risk ratios.
- 🔢 The impact of the odds ratio on sample size was discussed, showing that smaller odds ratios require larger sample sizes for significance.
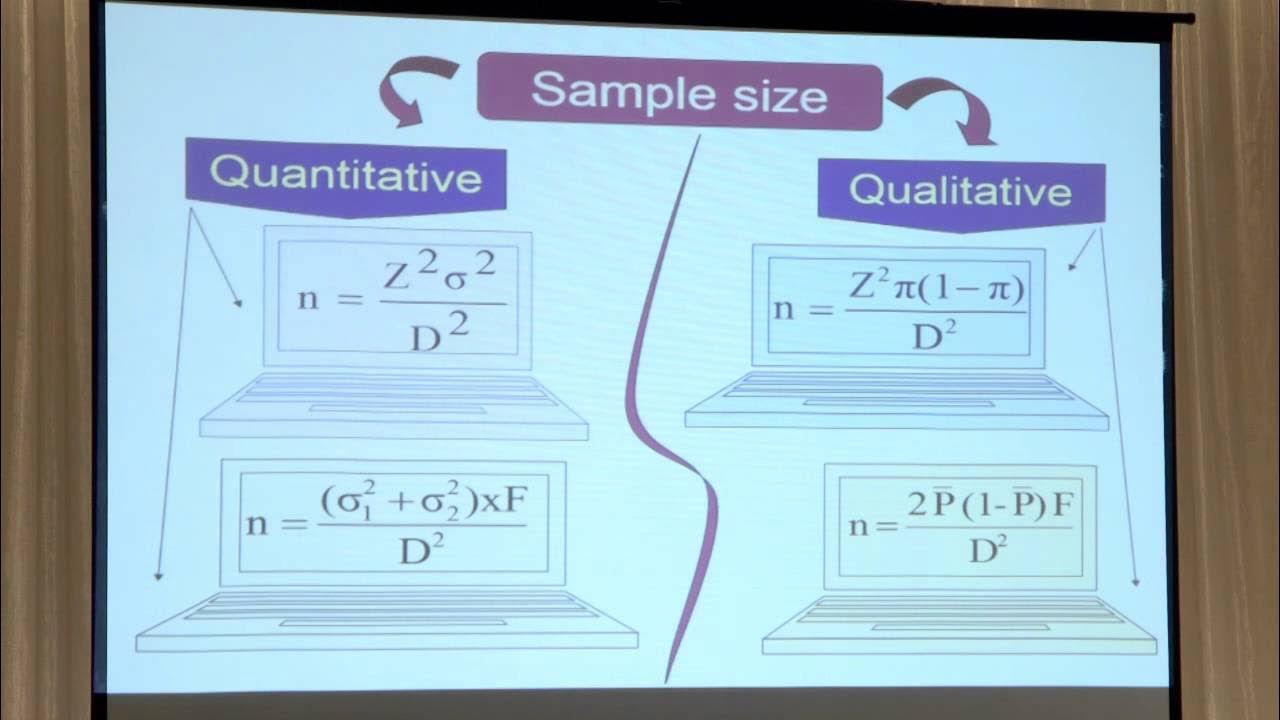
- 📐 The session provided formulas and examples for calculating sample size, emphasizing the importance of input variables such as odds ratio, population exposure proportion, and significance level.
- 🏥 The practical application of case-control studies was illustrated with real-world examples, including medical conditions and interventions.
Q & A
What is the main focus of today's session on case control studies?
-The main focus of the session is to discuss issues related to sample size in case control studies, not going into detail about the methodology or how it differs from cohort or cross-sectional studies.
What is the purpose of discussing neonatal jaundice and its potential effect on IQ in childhood?
-The purpose is to use it as a hypothetical example to illustrate the process of formulating a hypothesis and choosing a study design like case control, cross-sectional, or cohort to test it.
Why is it important to differentiate between exposure and outcome when planning a study?
-Differentiating between exposure and outcome is crucial because it helps in correctly identifying the study design and in dissecting the research question, which simplifies the planning process.
What is the fundamental difference between a cohort study and a case control study in terms of how they start?
-A cohort study starts with the exposure and measures the outcome later, while a case control study starts with the cases (those with the outcome) and then looks back to measure the exposure.
Why might a case control study be chosen over a cohort study or a randomized control trial?
-A case control study might be chosen when a clinical trial is not feasible due to resource constraints, long follow-up times, ethical concerns, or when the disease is rare and takes a long time to develop.
What are the limitations of using hospital-based controls in a case control study?
-Hospital-based controls may not accurately represent the exposure distribution in the general population, which can lead to biases. Community-based controls are often preferred for a more accurate representation.
Why is it essential to have a good pre-existing database for a case control study?
-A good pre-existing database is essential to ensure accurate and complete records of both cases and controls, which helps reduce biases and provides a more reliable basis for analysis.
What is the measure of association used in a case control study?
-The measure of association used in a case control study is the odds ratio, which compares the odds of exposure among cases with the odds of exposure among controls.
Can you explain the difference between odds ratio and risk ratio?
-The odds ratio compares the odds of exposure in those with the disease to those without the disease. The risk ratio compares the risk (or probability) of disease in those exposed to the risk factor versus those not exposed. Odds ratios can be the same as risk ratios when the disease is rare, but they can differ when the disease is more common.
How does the presence of recall bias affect a case control study that relies on interviews for exposure data?
-Recall bias can affect a case control study by causing cases to remember exposure more vividly than controls, potentially skewing the data and leading to inaccurate estimates of the association between exposure and disease.
Outlines
📚 Introduction to Case Control Study Design
The session begins with an introduction to case control studies, distinguishing them from other study designs like cross-sectional and cohort studies. The focus is on understanding the sample size calculations and issues specific to case control studies. An interactive approach is encouraged, with participants asked to respond to questions in the chat box. The speaker uses a hypothetical study about neonatal jaundice and its potential impact on childhood IQ as an example to illustrate the concepts of exposure and outcome in study design.
🔍 Understanding Case Control Study Fundamentals
This paragraph delves deeper into the specifics of case control studies, emphasizing their retrospective nature and starting with cases to look back at exposure levels. It contrasts this with other study designs like cohort and randomized control trials, which are prospective. The speaker discusses scenarios where case control studies are particularly useful, such as when diseases are rare or when ethical concerns prevent other study designs. The importance of selecting appropriate controls to mitigate biases is highlighted, along with the potential for case control studies to provide valuable insights despite their inherent limitations.
📈 Calculating Odds Ratio in Case Control Studies
The speaker introduces the concept of the odds ratio, a measure of association used in case control studies, and explains how to calculate it using a two-by-two contingency table. The explanation includes the definition of odds, the difference between odds ratio and risk ratio, and the importance of understanding these concepts when interpreting study results. The paragraph also addresses potential biases like recall bias and the importance of having accurate records to minimize them.
📝 The Significance of Odds and Risk Ratios
This section explores the relationship between odds ratios and risk ratios, demonstrating how they can be numerically identical in certain conditions, such as when the disease is not very common. The speaker uses examples with different disease prevalences to illustrate how the odds ratio remains constant even when the number of controls increases, whereas the risk ratio can change. The importance of selecting a representative control group to ensure the validity of the odds ratio is emphasized.
🧐 Factors Influencing Sample Size in Case Control Studies
The paragraph discusses the determinants of sample size in case control studies, including the risk factor or intervention being studied, its distribution in the population, the desired power and statistical significance of the study, and the case to control ratio. It also touches on the importance of considering confounding factors and adjustments that may be necessary for factors like cluster sampling or response rates.
📉 The Impact of Odds Ratio on Sample Size
The speaker explains how the odds ratio influences the required sample size, showing that a smaller odds ratio necessitates a larger sample size to detect a significant effect. The paragraph provides a graphical representation from a WHO reference to illustrate this point, emphasizing that for odds ratios less than 2.5, the sample size requirement increases dramatically. The examples highlight the importance of considering the odds ratio when planning a study to ensure adequate power.
📚 Detailed Explanation of Sample Size Calculation
This paragraph provides a detailed explanation of how to calculate sample size for binary exposure in case control studies. The speaker presents the underlying formula used for calculation and explains the significance of each component in the formula, such as the ratio of controls to cases, the average proportion, and the effect size. The paragraph also discusses the importance of understanding the odds ratio and how it can be calculated from proportions.
📝 Practical Examples of Sample Size Calculation
The speaker provides practical examples of sample size calculation for case control studies, using real-world scenarios such as diabetic retinopathy and hepatitis C infection. The examples demonstrate how to apply the formulas and concepts discussed earlier in the session, showing the inputs required for sample size calculation and the resulting sample size needed for each study.
🔍 Adjusting Sample Size for Limited Cases
This paragraph addresses a common issue in case control studies where the number of cases is limited. The speaker explains how to adjust the sample size by increasing the number of controls to maintain study power. The explanation includes a formula for calculating the adjustment factor based on the number of controls per case, highlighting that there is a maximum benefit to increasing controls beyond a certain point.
📈 Addressing Continuous Exposure in Sample Size Calculation
While the session primarily focuses on binary outcomes, this paragraph briefly touches on situations where exposure is continuous, such as measuring carbon monoxide levels in blood. The speaker explains that in such cases, sample size should be calculated based on the mean to avoid losing the dose-response relationship and overestimating the sample size.
📝 Conclusion and Final Thoughts
The session concludes with a summary of key points and a reminder of the importance of understanding case control study design and sample size calculation. The speaker also addresses questions from participants, providing clarifications on topics such as control group purposes, defining controls, and the use of matched versus unmatched studies. The session ends with a poll and a reminder of the next session in March.
Mindmap
Keywords
💡Case Control Study
💡Sample Size
💡Odds Ratio
💡Exposure
💡Outcome
💡Cross-Sectional Study
💡Cohort Study
💡Randomized Control Trial (RCT)
💡Confounding Factors
💡Statistical Significance
💡Power of a Study
Highlights
Introduction to calculating sample size in case-control studies and understanding related issues.
Distinguishing between different study designs like case-control, cross-sectional, and cohort studies.
Clarification on the importance of identifying exposure and outcome in study design.
Detailed explanation of how to conduct a case-control study, emphasizing starting with cases.
Examples illustrating the selection of cases and controls and the calculation of odds ratios.
Discussion on the challenges and limitations of case-control studies, such as recall bias and selection of appropriate controls.
Importance of having a pre-existing database for effective case-control and retrospective cohort studies.
Calculation of odds and understanding the concept of odds ratio in a 2x2 contingency table.
Demonstration of how to calculate the risk ratio and the difference between odds ratio and risk ratio.
Use of stata software for sample size calculation with step-by-step guidance.
Impact of odds ratio on sample size requirements, with examples showing the relationship.
Explanation of the U-shaped curve and its implications on sample size based on the distribution of the risk factor.
Discussion on the importance of power and statistical significance in determining sample size.
Practical examples demonstrating the process of sample size calculation for case-control studies.
Strategies for adjusting sample size when there is a limitation in the number of available cases.
Considerations for calculating sample size in matched case-control studies and the use of correlation coefficients.
Addressing common questions and misconceptions about case-control study design and sample size calculation.
Concluding remarks summarizing key points and预告ing upcoming sessions on epidemiological concepts.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: