15.6e Structural Determination From All Spectra Example 5 | Organic Chemistry
TLDRThe video script presents a detailed analysis of a compound using various spectroscopic techniques. Starting with the mass spectrum, the molecular weight of 135 indicates the presence of nitrogen. The absence of a significant peak at 137 suggests the absence of chlorine or bromine. The IR spectrum reveals a broad single peak for NH, and peaks around 3000 cm⁻¹ for sp³ CH bonds. Aromatic carbon-carbon peaks are seen around 1500 cm⁻¹, and a carbon-oxygen double bond is identified by its unusual position between 1600 and 1650 cm⁻¹, indicating conjugation. The carbon-13 NMR spectrum confirms the presence of a benzene ring and a carbonyl group. The proton NMR spectrum, with a 2:1:2 ratio of hydrogens, suggests a mono-substituted benzene ring. The final structure is deduced to be an amide with a benzene ring and a methyl group, with the position of the methyl group and the carbonyl's IR peak further confirming the structure. The video is a comprehensive guide for students on spectral analysis in organic chemistry.
Takeaways
- 🔍 **Molecular Weight Analysis**: The molecular weight of 135 suggests the presence of nitrogen in the compound, as it's an odd number.
- 🚫 **Absence of Halogens**: No significant peak at 137 indicates the absence of chlorine or bromine in the molecule.
- 🔵 **Nitrogen Identification**: The presence of a peak corresponding to nitrogen (NH) in the mass spectrum confirms nitrogen is part of the compound.
- 🌊 **Hydrogen Attachment**: A single peak in the NMR suggests one hydrogen atom is attached to the nitrogen.
- 🔲 **Alkane and Aromatic Signals**: Peaks just left and right of 3000 cm⁻¹ in the IR spectrum indicate sp³ and sp² hybridized carbon-hydrogen bonds, respectively.
- 🔗 **Carbonyl Group**: A signal between 1600 and 1650 cm⁻¹ in the IR suggests a carbon-oxygen double bond, experiencing conjugation.
- 💠 **Aromatic Ring Confirmation**: Four signals in the 110 to 160 ppm range in the carbon-13 NMR confirm the presence of a benzene ring.
- 📊 **Integration Ratio**: A 2:1:2 ratio in the proton NMR indicates five hydrogen atoms, suggesting a mono-substituted benzene ring.
- 🔘 **Symmetry in Aromatic Signals**: The pattern of singlets and triplets in the proton NMR suggests symmetry in the aromatic region, typical for a mono-substituted benzene ring.
- 🔬 **Methyl Group Positioning**: The position of the methyl group in the H NMR spectrum helps determine its placement in the final structure.
- ⚙️ **Conjugation Effect**: The carbonyl peak in the IR at 1640 cm⁻¹ indicates additional conjugation with the benzene ring, affecting the stretching frequency.
Q & A
What is the first thing to look for in the mass spectrum when trying to identify a compound?
-The first thing to look for is the molecular ion peak, which provides the molecular weight of the compound.
What does an odd molecular weight suggest about the compound's composition?
-An odd molecular weight suggests the presence of nitrogen in the compound, as nitrogen is the only common element with an odd atomic mass that could contribute to an odd molecular weight.
How can you determine if halogens like chlorine or bromine are present in a compound from the mass spectrum?
-By looking for an M plus 2 peak, which would indicate the presence of a halogen like chlorine or bromine. If this peak is not significant, it can rule out the presence of these halogens.
What does the presence of a peak in the region just below 3000 cm⁻¹ in an IR spectrum typically indicate?
-A peak just below 3000 cm⁻¹ typically indicates the presence of an N-H bond, as the hydrogen attached to nitrogen in an amine group shows up in this region.
What is the significance of the region between 1500 and 1650 cm⁻¹ in an IR spectrum?
-This region is where aromatic carbon-carbon peaks are found. A peak in this area suggests the presence of an aromatic ring in the compound.
How does the carbonyl peak position in an IR spectrum help in identifying the type of compound?
-A carbonyl peak that appears between 1600 and 1650 cm⁻¹ suggests the presence of a carbon-oxygen double bond experiencing some conjugation, which could indicate an amide or similar structure.
What does the alkane region in a carbon-13 NMR spectrum indicate?
-The alkane region in a carbon-13 NMR spectrum indicates the presence of carbon atoms that are part of an alkane, which are saturated hydrocarbons.
How does the integration ratio in an H NMR spectrum help in determining the structure of an aromatic ring?
-The integration ratio, such as a 2:1:2 pattern, indicates the number of hydrogens contributing to the peaks, which can help identify the pattern of substitution on the aromatic ring, such as mono-substitution.
What is the significance of a broad singlet peak in an H NMR spectrum?
-A broad singlet peak often indicates a hydrogen atom that is attached to a nitrogen atom, which can be a useful clue when identifying amines or other nitrogen-containing compounds.
How can the position of a methyl group in the H NMR spectrum help determine its position relative to other functional groups?
-The position of a methyl group in the H NMR spectrum can provide clues about its proximity to electronegative atoms or groups, as it will shift to different regions based on its environment.
What role does conjugation play in the chemical shifts observed in both the IR and NMR spectra?
-Conjugation can significantly affect the chemical shifts observed. For example, an amide carbonyl group conjugated with a benzene ring will have a lower stretching frequency in the IR spectrum, and the position of a methyl group next to a conjugated system in the NMR spectrum can also be influenced by the extent of conjugation.
Outlines
🔍 Analyzing the Mass Spectrum and Identifying Nitrogen
The first paragraph discusses the process of analyzing a compound using various spectroscopic techniques. The focus is on determining the molecular weight from the mass spectrum, which is found to be 135, indicating the presence of nitrogen in the compound. The absence of significant peaks at 137 suggests the absence of chlorine or bromine. The paragraph also touches on the identification of an NH group, the presence of sp3 and sp2 CH bonds, and aromatic carbon-carbon peaks. An unexpected carbon-oxygen double bond is identified, which is experiencing conjugation, later revealed to be part of an amide. The carbon 13 NMR spectrum confirms the presence of a benzene ring and a carbonyl group. The proton NMR spectrum is summarized, with the identification of an NH hydrogen, aromatic hydrogens, and a methyl group. The paragraph concludes with the deduction that the nitrogen is bonded to a hydrogen and that the compound contains a benzene ring and a carbonyl group.
🧬 Piecing Together the Structure Based on Spectroscopy
The second paragraph delves into the process of deducing the structure of the compound using the information gathered from the various spectra. The presence of a nitrogen atom, a carbonyl group, a benzene ring, and a methyl group is established. The discussion focuses on the placement of these groups within the molecule, considering the remaining bonds each group has to form. The carbonyl and nitrogen groups are identified as potential central components of the molecule. Two possible structures are drawn, differing in the placement of the benzene ring and the methyl group. The paragraph explains how the position of the methyl group in the H NMR spectrum and the IR carbonyl peak's unusual low frequency, due to conjugation with the benzene ring, help determine the correct structure. The conclusion is that the correct structure is the one with the benzene ring on the left and the methyl group on the right, with the carbonyl group in the middle, conjugated with both the nitrogen's lone pair and the benzene ring.
Mindmap
Keywords
💡Mass Spectrum
💡IR (Infrared Spectroscopy)
💡Carbon 13 NMR
💡Proton NMR
💡Molecular Weight
💡Nitrogen
💡Aromatic Carbon-Carbon Bonds
💡Conjugation
💡Amide
💡Benzene Ring
💡Methyl Group
Highlights
The molecular weight of the compound is 135, indicating the presence of nitrogen.
No significant peak at 137, ruling out chlorine and bromine in the compound.
Presence of a nitrogen-hydrogen bond, with a single hydrogen attached to nitrogen indicated by a single peak.
The sp3 CH bonds appear just to the right and sp2 CH bonds just to the left of 3000 in the spectrum.
A carbon-oxygen double bond is present, experiencing some conjugation as indicated by its peak between 1600 and 1650.
A benzene ring and a carbonyl group are identified in the compound through the 13C NMR spectrum.
The H NMR spectrum shows a nitrogen bonded to one hydrogen, a benzene ring, and a carbonyl group.
The NH proton appears as a broad singlet at 7 ppm in the H NMR spectrum.
The aromatic region of the H NMR spectrum shows a 2:1:2 integration ratio, indicating a mono-substituted benzene ring.
The methyl group adjacent to the nitrogen is expected to appear in the 3 or lower range of the H NMR spectrum.
The carbonyl peak in the IR spectrum appears at 1640 cm-1, indicating additional conjugation with the benzene ring.
Two possible structures are drawn based on the spectra, but the first structure is favored due to the position of the methyl group and carbonyl IR peak.
The final structure is confirmed as having the benzene ring and carbonyl in the middle, with the nitrogen bonded to a hydrogen and the methyl group at the end.
The use of multiple spectroscopic techniques (mass spec, IR, 13C NMR, 1H NMR) provides complementary information to deduce the structure of the compound.
Integration of signals in the H NMR spectrum is crucial for determining the number of equivalent hydrogens in different environments.
The chemical shift of the NH proton and the position of the carbonyl IR peak provide key clues to the connectivity of the nitrogen and carbonyl groups.
The presence of a benzene ring is confirmed by the characteristic 2:1:2 integration pattern in the aromatic region of the H NMR spectrum.
The position of the methyl group and its chemical environment can help distinguish between different possible structures.
The level of conjugation around the carbonyl group, as evidenced by the IR peak position, can provide insights into the overall structure of the molecule.
Transcripts
Browse More Related Video

15.6d Structural Determination From All Spectra Example 4 | Organic Chemistry

14.3 Interpreting More IR Spectra | Organic Chemistry

14.5 Isotope Effects in Mass Spectrometry | Organic Chemistry

15.6c Interpreting NMR Example 3 | Organic Chemistry

15.6b Interpreting NMR Example 2 | Organic Chemistry
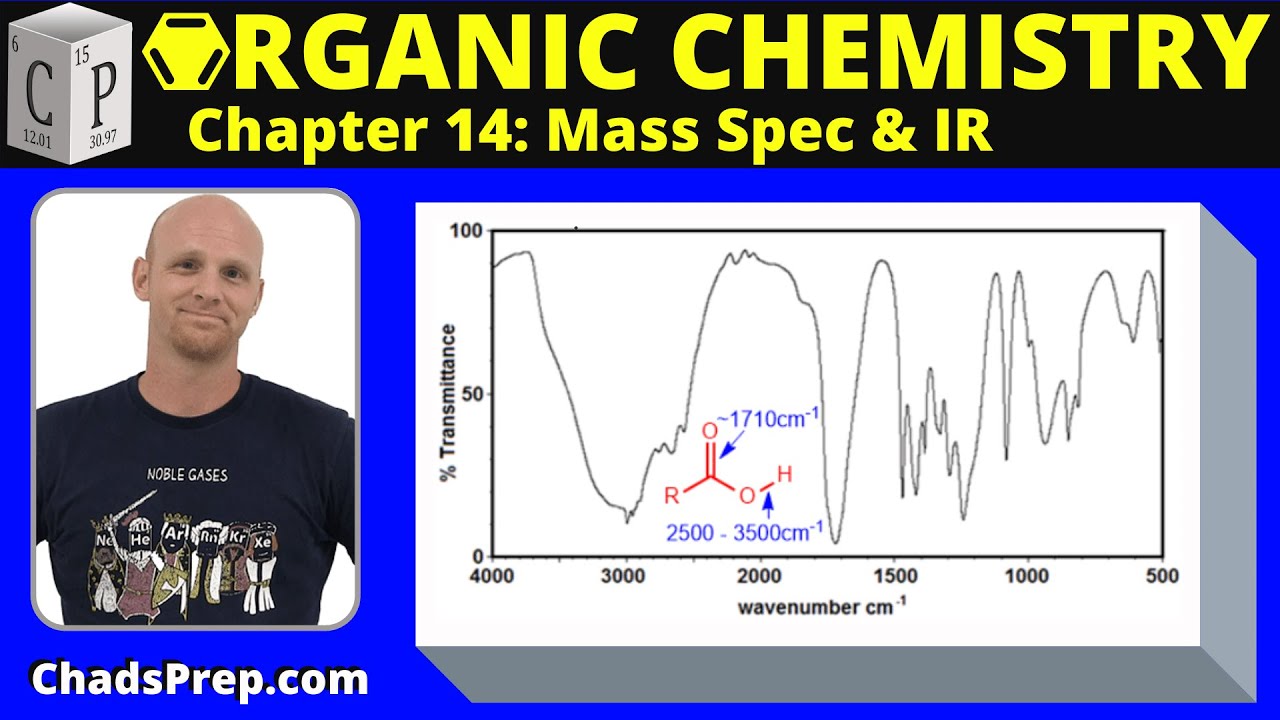
14.2a IR Spectra of Carbonyl Compounds | Organic Chemistry
5.0 / 5 (0 votes)
Thanks for rating: