14.5 Isotope Effects in Mass Spectrometry | Organic Chemistry
TLDRThe transcript discusses the interpretation of mass spectra in chemistry, focusing on the identification of elements within a compound. It explains how the presence of the M+1 peak at 73 can indicate the number of carbon atoms in a molecule, using the natural abundance of carbon-12 and carbon-13 isotopes. The video also covers how to quickly identify bromine or chlorine in a compound by examining the M+2 peak's intensity ratio. For instance, a 1:1 ratio suggests bromine, while a 3:1 ratio indicates chlorine. The summary emphasizes the efficient 10-second method for determining molecular weight and the presence of nitrogen, bromine, or chlorine from a mass spectrum, which is often combined with infrared or NMR spectra for further analysis.
Takeaways
- 🔬 **M+1 Peak Analysis**: The presence of an M+1 peak in a mass spectrum can help determine the number of carbon atoms in a compound, assuming the natural abundance of carbon isotopes is known.
- 🌟 **Carbon Isotope Abundance**: Carbon-12 is the most abundant isotope at 98.9%, with Carbon-13 at 1.1%, and Carbon-14 is negligible in most cases.
- 📊 **Relative Intensity Calculation**: By knowing the relative intensities of the molecular ion peak and the M+1 peak, one can calculate the number of carbons in a molecule using a specific formula.
- 🧮 **Empirical Formula Determination**: Using the M+1 peak and its intensity, one can deduce the empirical formula of a compound, such as C5H12 in the given example.
- 🚫 **Absence of M+2 Peak**: Typically, an M+2 peak is not observed in mass spectra unless the compound contains elements like bromine or chlorine, which have multiple isotopes.
- 🏷️ **Bromine Signature**: If an M+2 peak is present in a 1:1 ratio with the molecular ion peak, it is a strong indicator of bromine in the compound.
- 📈 **Chlorine Signature**: An M+2 peak in a 3:1 ratio with the molecular ion peak suggests the presence of chlorine in the molecule.
- 🔍 **Quick Identification**: The presence of odd molecular weights and specific M+2 peak ratios can quickly identify the presence of nitrogen, bromine, or chlorine in a compound.
- ⏱️ **10-Second Interpretation**: A rapid assessment of a mass spectrum can provide the molecular weight and indicate the presence of certain elements within seconds.
- 🧩 **Combining Spectra**: Mass spectra are often combined with infrared or NMR spectra for a more comprehensive analysis.
- 📚 **Educational Note**: Not all courses or professors cover the interpretation of M+1 and M+2 peaks, but it is a valuable skill found in most textbooks for mass spectrometry.
Q & A
What is the 'M+1 peak' in a mass spectrum and how is it useful?
-The 'M+1 peak' is a peak in a mass spectrum that appears at a mass one unit higher than the molecular ion peak (M peak). It is useful because it can indicate the presence of carbon-13 isotopes in the molecule, which can help in determining the number of carbon atoms in the compound.
How does the natural abundance of carbon isotopes affect the mass spectrum?
-The natural abundance of carbon-12 (98.9%), carbon-13 (1.1%), and the negligible amount of carbon-14 affect the mass spectrum by causing the M+1 peak to appear at a mass one unit higher than the molecular ion peak due to the presence of carbon-13 isotopes in some molecules.
What is the formula to determine the number of carbons in a compound using the M+1 peak intensity?
-The formula is to take the intensity of the M+1 peak, divide it by the intensity of the molecular ion peak, multiply by 100, and then divide by the natural abundance percentage of carbon-13 (1.1%). This will give you the number of carbon atoms in the compound.
How can the presence of bromine or chlorine in a molecule be identified from the mass spectrum?
-The presence of bromine or chlorine can be identified by the presence of an M+2 peak. If the M+2 peak is present in a 1:1 ratio with the molecular ion peak, it indicates bromine. If the ratio is approximately 3:1, it indicates chlorine.
Why is the M+2 peak a significant indicator for the presence of bromine or chlorine in a molecule?
-The M+2 peak is significant because bromine and chlorine have two major isotopes with different mass numbers. The presence of these isotopes in the molecule leads to two populations of molecules with different masses, resulting in an M+2 peak that is indicative of these elements.
What is the quick method to determine if a compound contains nitrogen based on the mass spectrum?
-If the molecular weight indicated by the molecular ion peak is an odd number, it suggests the presence of an odd number of nitrogen atoms in the compound, as nitrogen has an atomic mass of approximately 14.
How can the relative intensities of the molecular ion peak and the M+1 peak be used to find useful information?
-By knowing the relative intensities of these peaks, one can determine the number of carbon atoms in the compound using the provided formula, which is particularly useful when the exact structure of the compound is not immediately clear.
Why is it stated that the M+2 peak is not usually significant in most mass spectra?
-The M+2 peak is not usually significant because most elements, like carbon, have a single major isotope, making it unlikely to have two isotopes in the same molecule that would result in an M+2 peak. However, elements like bromine and chlorine, which have two major isotopes, can exhibit an M+2 peak.
What is the approximate ratio of bromine isotopes that leads to the M+2 peak?
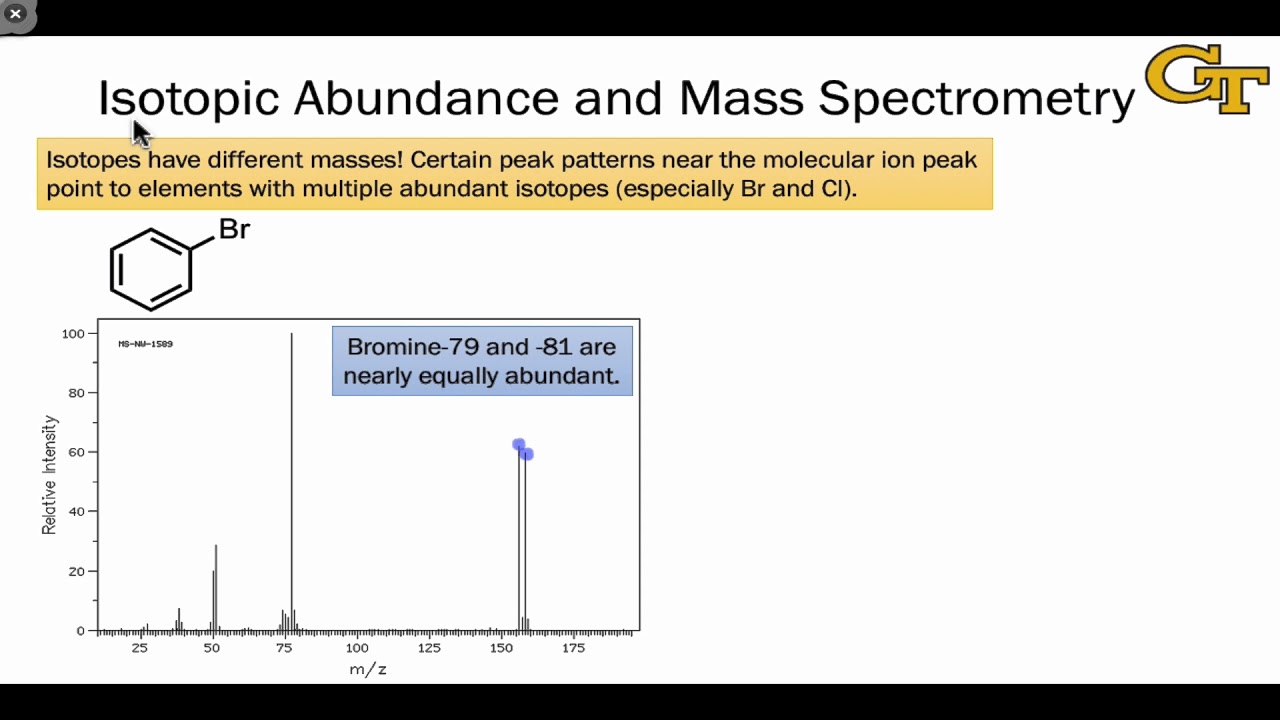
-The approximate ratio of bromine isotopes is 51% for bromine-79 and 49% for bromine-81, which leads to the presence of an M+2 peak due to the nearly equal abundance of the two isotopes.
How does the presence of chlorine in a molecule affect the mass spectrum?
-The presence of chlorine in a molecule results in two populations of molecules due to the two major isotopes of chlorine, chlorine-35 and chlorine-37. This leads to an M+2 peak with a 3:1 ratio, which is indicative of chlorine in the molecule.
What is the efficient 10-second interpretation of a mass spectrum?
-The efficient 10-second interpretation involves identifying the molecular ion peak to determine the molecular weight, checking if the molecular weight is odd to infer nitrogen presence, and looking for an M+2 peak to identify the presence of bromine or chlorine based on the peak ratio.
How can additional information from the mass spectrum be combined with other spectroscopic data?
-Additional information from the mass spectrum can be combined with infrared (IR) or nuclear magnetic resonance (NMR) spectra to provide a more comprehensive understanding of the molecular structure and functional groups present in the compound.
Outlines
🧬 Understanding Carbon Isotopes in Mass Spectra
This paragraph discusses the use of the M+1 peak in a mass spectrum to determine the number of carbon atoms in a compound. It explains that carbon-12 is the most abundant isotope, with carbon-13 present at 1.1%. The presence of carbon-13 can cause an M+1 peak at a mass one unit higher than the molecular ion peak. By knowing the relative intensities of the molecular ion and M+1 peaks, one can calculate the number of carbon atoms in the compound using a simple formula. The example given concludes that the compound has five carbon atoms, resulting in an empirical formula of C5H12. The paragraph also touches on the rarity of an M+2 peak, except in the case of elements like bromine and chlorine, which have two major isotopes leading to distinct M+2 peaks in their mass spectra.
🔍 Rapid Identification of Elements in Mass Spectra
The second paragraph focuses on the quick identification of elements such as nitrogen, bromine, and chlorine using mass spectra. It emphasizes that the molecular weight can be determined in less than 10 seconds by finding the parent peak. If the molecular weight is an odd number, it indicates the presence of an odd number of nitrogen atoms. The presence of an M+2 peak can help identify bromine or chlorine in the compound. A 1:1 ratio of the M+2 peak to the parent peak suggests bromine, while a 3:1 ratio indicates chlorine. This method provides an efficient way to interpret mass spectra in a short amount of time, with the possibility of combining this information with infrared or NMR spectra for a more comprehensive analysis.
Mindmap
Keywords
💡Spectrum
💡M+1 Peak
💡Natural Abundance
💡Carbon-12, Carbon-13, Carbon-14
💡Relative Intensities
💡Empirical Formula
💡Molecular Formula
💡M+2 Peak
💡Bromine and Chlorine Isotopes
💡Odd Number of Nitrogen
💡Efficient 10-Second Interpretation
Highlights
The m+1 peak in a spectrum can provide useful information about the number of carbon atoms in a compound.
The presence of carbon-12, carbon-13, and carbon-14 can affect the mass of a molecule and the resulting spectrum.
Carbon-13 can cause a molecule to weigh one unit more, creating an m+1 peak at 73 if the base molecular weight is 72.
Relative intensities of the molecular ion peak and the m+1 peak can be used to determine the number of carbons in a compound.
A quick formula is provided to calculate the number of carbon atoms using the intensities of the peaks.
The empirical formula or molecular formula can be deduced from the mass of the m+1 peak and the number of carbon atoms.
The presence of an M plus two peak is unusual and can indicate the presence of bromine or chlorine in a molecule.
Bromine has two isotopes, bromine-79 and bromine-81, which are roughly in a 50/50 ratio.
The presence of bromine is indicated by two peaks in a 1:1 ratio, which is a clear giveaway in a mass spectrum.
Chlorine has two major isotopes, chlorine-35 and chlorine-37, with a natural abundance ratio of approximately 3:1.
An M plus two peak in a 3:1 ratio is a strong indicator of chlorine being present in the molecule.
The molecular weight and the presence of nitrogen can be quickly determined from the mass spectrum.
An odd molecular weight often suggests the presence of an odd number of nitrogen atoms.
The absence of an M plus two peak indicates the absence of bromine or chlorine in the compound.
Combining the mass spectrum with infrared or NMR spectra can provide additional information for compound identification.
A 10-second efficient interpretation of a mass spectrum can provide key information about the compound's composition.
The transcript provides a method to quickly interpret mass spectra and identify elements like bromine and chlorine.
Transcripts
Browse More Related Video
13.04 Isotopic Abundance in Mass Spectrometry

15.6e Structural Determination From All Spectra Example 5 | Organic Chemistry

15.6d Structural Determination From All Spectra Example 4 | Organic Chemistry

15.6a Interpreting NMR Example 1 | Organic Chemistry

15.5a The Chemical Shift in C 13 and Proton NMR | Organic Chemistry

15.6c Interpreting NMR Example 3 | Organic Chemistry
5.0 / 5 (0 votes)
Thanks for rating: