Basics of mass Spectroscopy. How to estimate the formula from molar mass. (M+ and M+1, M+2 )
TLDRThis educational video introduces the fundamental principles of mass spectrometry, focusing on its relevance to sophomore organic chemistry without delving into excessive detail. It explains how mass spectrometry separates ions based on their mass-to-charge ratio using a magnetic field, emphasizing that only charged particles are detected. The video also covers the formation of radical cations from molecules upon electron bombardment, the significance of molecular ion peaks, and how different isotopes contribute to the mass spectrum. It further explores the calculation of molecular formulas from mass spectrometry data and touches on fragmentation patterns and the identification of specific elements within compounds, making it an essential primer for students embarking on their study of organic chemistry.
Takeaways
- 🧲 Mass spectroscopy is a technique that ionizes chemical compounds to determine their mass and structural information.
- 🔬 The process involves applying a magnetic field to charged particles, which deflect based on their mass, allowing for mass determination.
- 💥 The molecular ion or parent ion is created by electron bombardment, resulting in the loss of an electron and forming a radical cation.
- 🌀 The molecular ion is often unstable and further fragments into a more stable carbocation and a radical.
- 📈 The base peak in a mass spectrum has an intensity of 100%, with other peaks having relative intensities based on the base peak.
- 📊 The m/z (mass-to-charge) ratio of the ions provides information about the molecular weight and structure of the compound.
- 📈 The presence of isotopes, such as carbon-13, can be used to calculate the empirical formula of the compound by analyzing the m+1 and m+2 peaks.
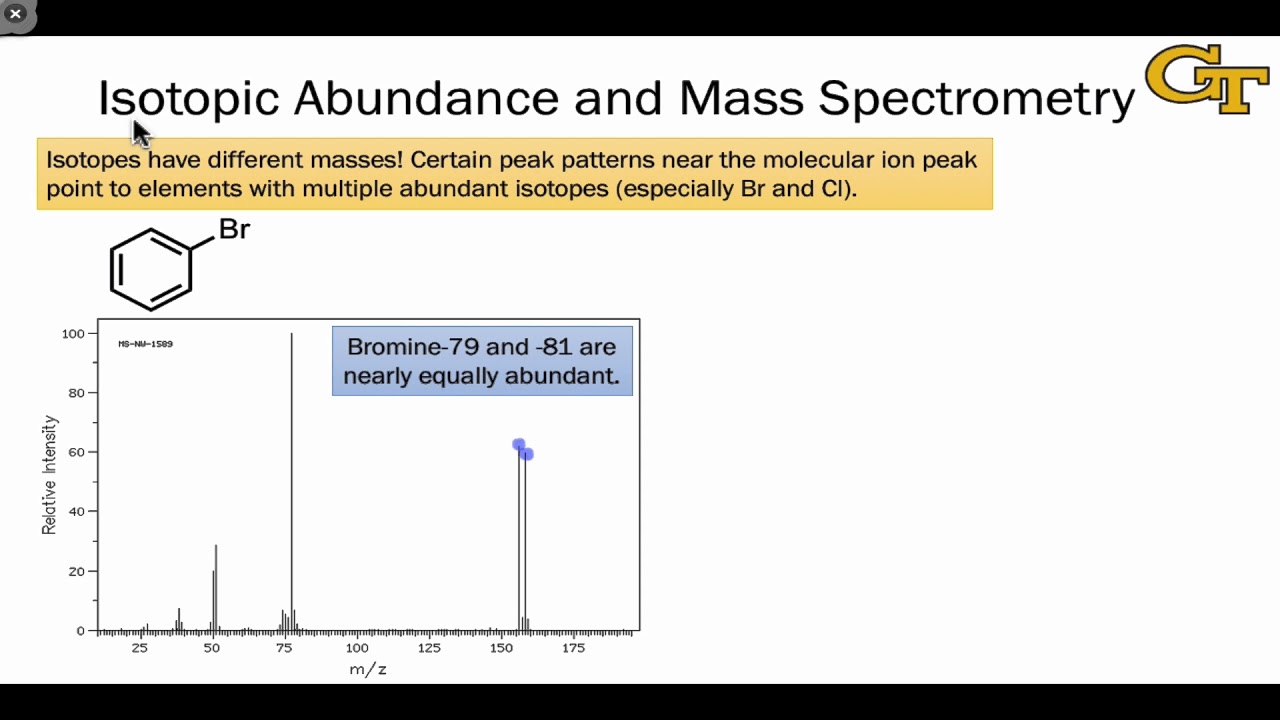
- 🔍 The mass spectrum can reveal the presence of specific functional groups or elements, such as halogens like bromine or chlorine, through characteristic peak patterns.
- 🧬 For compounds with multiple halogens, the relative heights of the m+n peaks can indicate the number and type of halogens present.
- 🔧 The process of determining a compound's formula from mass spectroscopy data involves comparing peak heights and using the known isotopic abundances.
- 📝 Understanding mass spectroscopy data is a valuable tool in organic chemistry for identifying and characterizing compounds.
Q & A
What principle does mass spectrometry rely on for separating ions?
-Mass spectrometry relies on the principle that a magnetic force will act on charged particles, such as cations and anions, allowing for their separation based on mass-to-charge ratio.
Which types of electrons are most likely to be ejected to form a radical cation in mass spectrometry?
-The electrons most likely to be ejected to form a radical cation are those that are very loosely bound to the molecule, typically the pi bond electrons or the non-bonding electrons from lone pairs.
Why do radical cations, rather than neutral radicals or uncharged molecules, show up in mass spectrometry?
-Radical cations show up in mass spectrometry because the technique relies on the deflection of charged particles in a magnetic field, and neutral radicals or uncharged molecules do not experience this deflection.
What happens to a molecule's radical cation in mass spectrometry if it's unstable?
-If a molecule's radical cation is unstable, it will typically break down further into smaller fragments, such as a carbocation and a radical, with the carbocation being detected in the mass spectrometer.
What is the significance of the 'base peak' in a mass spectrum?
-The 'base peak' in a mass spectrum represents the most abundant fragment ion and is assigned an intensity of 100%. It indicates the most stable carbocation fragment produced during the ionization process.
How can the presence of an M+1 peak in a mass spectrum be used to infer molecular structure?
-The M+1 peak arises from isotopes with one extra neutron, such as carbon-13. Its relative intensity compared to the M peak can be used to estimate the number of carbon atoms in the molecule.
What does an M+2 peak indicate in a mass spectrum?
-An M+2 peak often indicates the presence of isotopes or elements with two additional neutrons, such as chlorine or bromine, providing clues about the molecular structure and the presence of certain halogens.
How does the 'even nitrogen rule' assist in determining molecular formula from mass spectrometry data?
-The 'even nitrogen rule' suggests that molecules with an even molar mass will contain an even number of nitrogen atoms, helping to narrow down the possible molecular formulas.
How can fragmentation patterns help in identifying molecular structure in mass spectrometry?
-Fragmentation patterns, such as the base peak and the presence of specific fragment ions, can reveal information about the molecule's structure, including functional groups and the stability of potential fragments.
What is the significance of the ratio of the heights of M+ and M+2 peaks in identifying halogen presence?
-The ratio of the heights of M+ and M+2 peaks can indicate the presence of halogens like chlorine or bromine. For example, a 1:1 ratio suggests bromine, while a 3:1 ratio suggests chlorine, based on their isotopic abundance.
Outlines
🔬 Introduction to Mass Spectrometry
This section introduces mass spectrometry, focusing on its basic principles without delving into overly complex details, making it suitable for sophomore organic chemistry students. The fundamental concept discussed is the role of magnetic force in mass spectrometry, which affects charged particles such as cations and anions, with an emphasis on the creation of radical cations through electron bombardment. The process involves the loss of loosely bound electrons, typically from pi bonds or lone pairs, leading to the formation of a radical cation or a parent ion. This initial step is crucial for the mass spectrometry process, as only charged particles are detected, highlighting the importance of the type of bonds present in molecules and the resulting charged species.
🧪 Basic Mechanisms and Peaks in Mass Spectrometry
This paragraph dives deeper into the mechanisms of mass spectrometry, specifically how molecular ions break down into more stable carbocations, resulting in the formation of base peaks on the mass spectrum. The base peak, which is the most intense peak representing the most stable fragment, plays a crucial role in understanding mass spectrometry data. The discussion includes the process of fragmentation, the significance of the magnetic field in separating charged particles, and the basics of interpreting molecular ion peaks, including M+ (parent ion peak) and M+1 peaks. The M+1 peak, which results from the presence of carbon-13 isotopes, is particularly emphasized for its role in determining the molecular formula of the compound being analyzed.
📊 Predicting Molecular Formulas and Fragmentation Patterns
This section focuses on the methodology for predicting molecular formulas based on mass spectrometry data, exploring the impact of different atomic compositions such as carbon, hydrogen, nitrogen, and oxygen on the molar mass. It introduces the concept of the nitrogen rule for determining the number of nitrogen atoms based on the molar mass's parity and discusses the potential molecular formulas that could correspond to a given molar mass. Additionally, it explains how the presence of oxygen and nitrogen affects the formula calculation and the concept of the index of hydrogen deficiency, which helps predict the structure's complexity, including rings and double bonds. This part provides a comprehensive approach to deciphering mass spectrometry data for molecular formula prediction, emphasizing the importance of considering all possible atomic compositions.
🔍 Advanced Analysis and Interpretation Techniques
This paragraph delves into more sophisticated techniques for analyzing and interpreting mass spectrometry data. It focuses on the calculation and significance of M+1 and M+2 peaks, which offer insights into the molecule's carbon content through the relative abundance of carbon-13. This segment explains how to use the ratio of these peaks to estimate the number of carbon atoms and, consequently, other elements in the molecule. It also touches upon the possibility of identifying the presence of oxygen by considering the excess of hydrogen atoms and their impact on the molecular formula. This advanced analysis underscores the utility of mass spectrometry in determining the precise chemical composition of a molecule through a combination of peak ratio calculations and elemental analysis.
🧐 Identifying Halogens and Fragmentation Patterns
The discussion shifts towards identifying halogens in organic compounds using mass spectrometry, particularly focusing on bromine and chlorine's unique isotopic patterns that result in distinctive M+2 peaks. This part explains how these patterns can help identify the presence of bromine or chlorine in a molecule, including the 50/50 isotopic distribution of bromine that leads to equal M+ and M+2 peak heights and the 3:1 ratio of chlorine isotopes that affects the peak heights differently. It also explores how these halogen patterns are reflected in the fragmentation process, providing clues about the molecular structure, such as the formation of specific carbocations after halogen loss. This section underscores the nuanced interpretation of mass spectrometry data in detecting halogens and understanding molecular fragmentation.
🧬 Special Cases in Halogen Identification
This final paragraph discusses special cases in identifying halogenated compounds with mass spectrometry, focusing on molecules containing multiple bromines. It describes the characteristic peak patterns observed when a compound contains two bromine atoms, leading to a distinctive 1:2:1 ratio of M+, M+2, and M+4 peaks due to the combinations of bromine isotopes. This unique pattern aids in the identification of di-brominated compounds and demonstrates the complexities of interpreting mass spectrometry data for molecules with various isotopic compositions. This segment highlights the detailed analytical approach required to deduce the chemical structure of complex halogenated organic molecules through mass spectrometry.
Mindmap
Keywords
💡Mass Spectroscopy
💡Radical Cation
💡Base Peak
💡Fragmentation
💡Molecular Ion
💡M Plus One Peak
💡Isotopes
💡Magnetic Field
💡Ionization
💡Sigma Bonds
Highlights
Basic concepts of mass spectroscopy are introduced, providing foundational knowledge for organic chemistry students.
Mass spectroscopy works on the principle of applying magnetic force on charged particles, typically resulting in the detection of cations and radical cations.
The creation of a radical cation, also known as an apparent ion, involves electron bombardment and the loss of an electron from the molecule.
Electrons that are loosely bound to molecules, such as pi bond electrons or lone pairs, are more likely to be lost during ionization.
The molecular ion or radical cation is typically unstable and may further fragment into a carbocation and a radical.
The base peak in a mass spectrum has an intensity of 100%, with other peaks having intensity relative to it.
The mass spectroscopy process involves charged ions passing through a magnetic field, which causes them to deflect and allows for the determination of mass based on deflection.
Molecular ion peaks, including the parent ion and isotopes, can be used to determine the molar mass and predict the molecular formula of a compound.
The presence of carbon isotopes (carbon-12, carbon-13) can be identified by the m+1 peak in the mass spectrum.
The method for calculating the number of carbons in a compound using the m+1 peak and the relative abundance of carbon-13 is explained.
The even-carbon rule for nitrogen atoms and odd-mass rule for nitrogen atoms in the context of mass spectroscopy are discussed.
The impact of adding nitrogen or oxygen atoms to a compound's formula and how it affects the hydrogen deficiency index is explored.
The technique for determining the number of carbons in a molecule using the m+1 and m+2 peaks of isotopes like bromine is described.
The identification of bromine in a compound through the examination of m+2 and m+4 peaks and their relative heights is detailed.
The recognition of chlorine in an alkyl halide through the analysis of m+1 and m+2 peaks and their ratios is explained.
The process of calculating the molecular formula of a compound using mass spectroscopy data, including the consideration of isotopes and peak ratios, is outlined.
The practical application of mass spectroscopy in organic chemistry for determining the structure and composition of molecules is emphasized.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: