Using Logarithms and Natural Logarithms in Chemistry
TLDRThis video script offers a comprehensive review of logarithms and natural logarithms, essential mathematical concepts in chemistry. It explains how logarithms are another representation of exponents and emphasizes the importance of understanding the conversion between logarithmic and exponential forms. The script focuses on base-10 logarithms, which are standard in chemistry and on calculators. It demonstrates how to calculate pH, a common application in chemistry, and how to manipulate logarithmic equations, including taking logs of fractions and canceling out logs to revert to exponential form. The video also covers the use of anti-logs and the properties of logarithms, such as the inability to take logs of zero or negative numbers. It concludes with examples of solving for variables in logarithmic equations and encourages viewers to practice with additional problems for a deeper understanding of the subject.
Takeaways
- 📚 **Understanding Logarithms**: Logarithms (logs) and natural logarithms (ln) are fundamental in Chemistry, often used in formulas for acids, bases, and buffers.
- 🔁 **Conversion Between Forms**: It's crucial to know how to switch between logarithmic and exponential forms, which is frequently required in Chemistry problems.
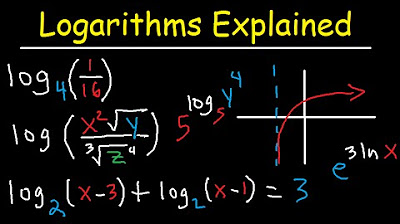
- 🔢 **Base 10 Logs**: Chemistry typically uses base 10 logarithms, which is also what most calculators are set to, so the base is often implicit in formulas.
- ⚖️ **pH Calculation**: The pH of an acid is calculated using the formula pH = -log[acid concentration], which involves using a calculator in scientific notation mode.
- 📉 **Logarithms of Fractions**: When taking the log of a fraction, ensure to perform operations within the parentheses before applying the log.
- 📈 **Natural Logarithms**: The process of taking a natural log (ln) is similar to taking a log, but uses the base e instead of base 10.
- ✅ **Cancelling Logs**: To cancel out a log, take the anti-log (base 10) of both sides of the equation, which simplifies the expression.
- 🔍 **Solving for Variables**: When given a pH value, you can find the acid concentration by taking the anti-log and using the formula [acid] = 10^(-pH).
- 🚫 **Logarithm Rules**: You cannot take the log or natural log of zero or a negative number, as these operations are undefined and will result in a calculator error.
- ➗ **Properties of Logs**: When a number with an exponent is logged, the exponent comes down in front of the log, which is useful for solving equations in exponential form.
- 🔗 **Further Practice**: Additional examples and practice problems are available through links provided in the description for better preparation in Chemistry classes.
Q & A
What is the relationship between logarithms and exponents?
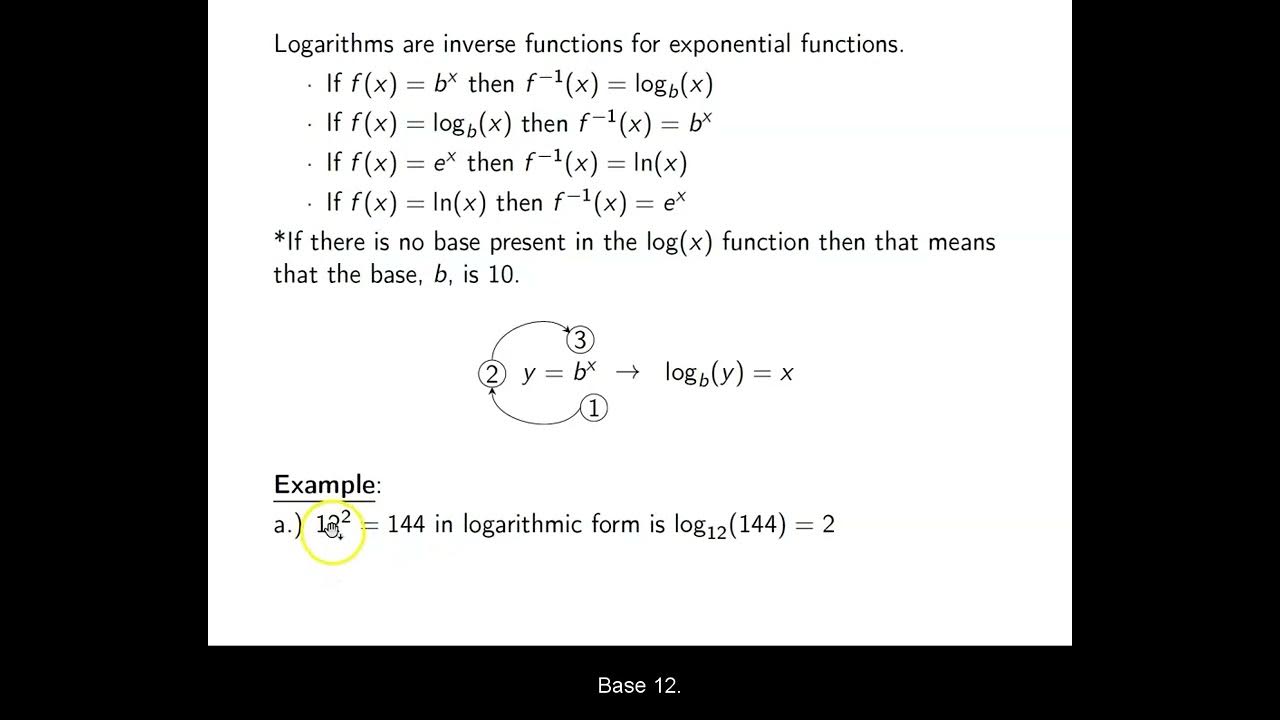
-Logarithms are the inverse operation to exponentiation. They are another way to think about exponents, allowing us to convert exponential form to logarithmic form and vice versa.
What is the generic form of a logarithm called?
-The generic form of a logarithm is called logarithmic form, and it is used to express the relationship between the base, the result of exponentiation, and the exponent.
What is the base of logarithms typically used in Chemistry?
-In Chemistry, the base of logarithms typically used is 10, which is also the base that most calculators are designed to work with.
How do you find the pH of an acid concentration?
-The pH of an acid concentration is found using the formula pH = -log[acid concentration]. You plug in the value and use a calculator to compute the logarithm.
What is the significance of taking the log of a fraction in Chemistry?
-Taking the log of a fraction is important in Chemistry, especially when finding the pH of a buffer. It helps in simplifying the calculation and applying logarithmic properties.
How do you take the natural log (ln) of a value?
-Taking the natural log of a value is similar to taking the log base 10. You perform any operations inside parentheses first, then take the ln of the value, and continue with the rest of the formula.
What is the process to cancel out a log with a base of 10?
-To cancel out a log with a base of 10, you take the anti-log (base 10) of both sides, which cancels the logs and leaves you with an exponent.
How do you convert a pH value back to acid concentration?
-To convert a pH value back to acid concentration, you use the formula [acid] = 10^(-pH). You input the pH value as a negative exponent on your calculator to find the concentration.
What is the process to cancel out a natural log in an equation?
-To cancel out a natural log in an equation, you raise both sides of the equation by the base e. This cancels out the ln and leaves you with the exponential form.
Why can't you take the log or natural log of zero or a negative number?
-Logarithms and natural logarithms are undefined for zero and negative numbers because you cannot have a number as an exponent that results in zero or a negative value.
What happens when you take the log or ln of a number with an exponent?
-When you take the log or ln of a number with an exponent, the exponent comes down in front of the log or ln, allowing you to manipulate the equation further.
How can you use logarithms to solve for a variable in an equation?
-You can use logarithms to solve for a variable by isolating the variable term and then applying logarithmic properties to simplify the equation and solve for the variable.
Outlines
🧮 Understanding Logarithms and Natural Logarithms in Chemistry
This paragraph introduces the concept of logarithms and natural logarithms, which are essential in Chemistry. It explains that logarithms are another way to express exponents, and provides the generic forms of logarithmic and exponential expressions. The paragraph emphasizes the use of base 10 logarithms in Chemistry and shows how to switch between logarithmic and exponential forms. It also demonstrates how to calculate the pH of an acid concentration using a logarithm, and how to handle logarithms of fractions, which is important when finding the pH of a buffer. Additionally, it covers the process of taking natural logarithms and the method to cancel out logs or natural logs to revert to exponential form. The explanation includes practical steps on how to use a scientific calculator to perform these operations.
🚫 Logarithmic Rules and Properties in Chemistry
The second paragraph outlines important rules and properties related to logarithms and natural logarithms that are crucial for Chemistry. It states that logarithms and natural logarithms cannot be taken of zero or negative numbers, which would result in an error on a calculator. The paragraph also explains that when taking the logarithm of a number with an exponent, the exponent must come down in front of the logarithm. It provides an example of solving for a variable in an exponential form by taking the natural logarithm of both sides, which allows the exponent to be isolated and solved. The summary also touches on the application of these concepts in Chemistry 2, particularly in finding rate laws, and encourages further practice for better preparation.
Mindmap
Keywords
💡Logarithms
💡Natural Logarithms
💡Logarithmic Form
💡Exponential Form
💡Base 10
💡pH
💡Buffer
💡Anti-log
💡Scientific Notation
💡Logarithmic Rules and Properties
💡Rate Law
Highlights
Logarithms and natural logarithms are integral to many formulas in Chemistry.
Logarithms are another way to think about exponents and can be converted to exponential form.
In Chemistry, logarithms are typically base 10, which is also the base used by calculators.
The pH formula is an example of a logarithmic formula used in Chemistry to find the acidity of a solution.
To calculate pH, use the formula pH = -log(acid concentration) and a scientific calculator.
Taking the log of a fraction is a common task when finding the pH of a buffer in Chemistry 2.
Natural logarithms (ln) are used similarly to logarithms but with the base e.
Canceling out a log involves taking the anti-log (base 10) of both sides of the equation.
The anti-logarithm of pH can be found using the formula acid concentration = 10^(-pH).
Canceling out a natural log involves raising both sides of the equation by the base e.
Logarithms cannot be taken of zero or negative numbers, which is a useful check for calculation errors.
The exponent of a number in logarithmic form comes down in front of the log when using properties of logs.
The natural log can be used to solve for variables in exponential forms by isolating the variable.
In Chemistry 2, the natural log is often used when determining the rate law.
The transcript provides examples of how to use logarithms and natural logarithms in Chemistry.
Additional practice problems and resources are available through a link in the description for further preparation.
The video is part of a playlist that guides viewers through various Chemistry concepts.
Transcripts
5.0 / 5 (0 votes)
Thanks for rating: