Translating Chemistry Word Problems
TLDRThe video script provides a comprehensive guide to solving chemistry word problems by translating them into mathematical operations. It emphasizes the importance of recognizing keywords that signify addition, subtraction, multiplication, and division. The script also covers the identification of states of matter in chemical equations, such as aqueous, liquid, solid, and gas, and how to denote them. It explains the process of writing balanced chemical equations by identifying reactants and products, and understanding the types of chemical reactions, including decomposition, single replacement, combustion, and precipitation reactions. The guide further delves into dilution problems and molarity, illustrating how to use the word 'of' to associate volumes with their respective molarities and concentrations. The video encourages practice and provides resources for further learning.
Takeaways
- 📚 Chemistry word problems can be translated using specific keywords that correspond to mathematical operations.
- ➕ Keywords for addition include 'combines with', 'the sum', 'increased by', 'plus', 'the total of', and 'and'.
- ➖ Keywords for subtraction are 'the difference of', 'minus', 'decreased by', 'subtracted from', 'less than', and 'reduced by'.
- ✖️ Multiplication is often indicated by 'the product of', 'times', or simply the word 'of'.
- ➗ Division is signified by 'the ratio of' or 'the quotient of'.
- ➡️ Words like 'form', 'produce', 'create', 'yield', and 'result' are used to represent an equal sign or an arrow in a chemical equation.
- 🔍 In chemical equations, the state of a compound (aqueous, liquid, solid, gas) is indicated by words like 'dissolved', 'precipitate', 'vapor', and 'gaseous'.
- ➳ 'Reacts with' or 'combines with' are keywords for reactants, while 'forms', 'yields', 'creates', 'results in', 'converts into', and 'produces' are for products.
- 🔄 Types of chemical reactions are hinted by words like 'decomposes' (decomposition), 'rust' (single replacement), 'combusts' or 'burns' (combustion), and 'precipitate' (precipitation).
- 🧪 Balancing chemical equations involves identifying reactants and products, using the correct states and reaction types.
- 💧 In dilution problems, 'of' connects the initial molarity and volume (M1 and V1), and helps to identify what you are solving for (M2 and V2).
- 🔢 The word 'of' is also crucial in molarity and solution stoichiometry to associate the correct volume with the correct solution concentration.
Q & A
What are some common keywords and phrases that indicate addition in a chemistry word problem?
-Keywords and phrases that indicate addition include 'combines with', 'the sum', 'increased by', 'plus', 'the total of', and the word 'and'.
How can you identify subtraction in chemistry word problems by keywords or phrases?
-Subtraction can be identified by keywords and phrases such as 'the difference of', 'minus', 'decreased by', 'subtracted from', 'less than', and 'reduced by'.
What does the word 'of' typically represent in the context of chemistry word problems?
-In chemistry word problems, the word 'of' often indicates multiplication and can be a hint to that operation.
What are some keywords that can help identify the state of a compound or element in a chemical equation?
-Keywords that can help identify the state include 'aqueous', 'liquid', 'solid', 'gaseous', 'vapor', 'diatomic molecules', 'dissolved', 'dissociated', 'ionized', and 'precipitate'.
How can you determine the reactants and products in a chemical equation from a word problem?
-Reactants can be determined by keywords such as 'reacts with' or 'combines with', while products are indicated by words like 'forms', 'yields', 'creates', 'results in', 'converts into', or 'produces'. Reactants are on the left side of the reaction arrow, and products are on the right side.
What does the keyword 'decomposes' indicate about the type of chemical reaction being described?
-The keyword 'decomposes' indicates a decomposition reaction, where a compound breaks down into multiple products.
How does the keyword 'rust' relate to a specific type of chemical reaction?
-The keyword 'rust' is a hint that the reaction being described is a single replacement reaction, where one element replaces another in a compound to form a new compound and an element.
What type of reaction is indicated when the keywords 'combusts' or 'burns' are used in a word problem?
-The keywords 'combusts' or 'burns' indicate a combustion reaction, where a reactant reacts with oxygen, typically producing carbon dioxide and water.
What is the significance of the word 'precipitate' in the context of a chemical reaction?
-The word 'precipitate' signifies a precipitation reaction, where reactants in an aqueous state form a solid, which is the precipitate.
How can the word 'of' help in setting up a dilution problem in chemistry?
-The word 'of' helps to associate the correct volume with the correct solution concentration in a dilution problem, indicating multiplication between the initial molarity and volume (M1V1) and the final molarity and volume (M2V2).
What role does molarity (represented by M) play in dilution and molarity questions in chemistry?
-Molarity (M) represents the concentration of a solution in moles per liter. It is a key factor in dilution problems, where it is used to calculate the volumes of solutions when given their concentrations and the desired concentration after dilution.
How can understanding the keywords in a chemistry word problem assist in solving it?
-Understanding the keywords in a chemistry word problem helps to identify the type of reaction, the states of the compounds or elements, the reactants and products, and the specific operations (like addition, subtraction, multiplication, or division) that need to be performed to solve the problem.
Outlines
🔍 Understanding Chemistry Word Problems
This paragraph explains the importance of translating word problems in chemistry, which can be challenging due to the specialized language. It covers common keywords for mathematical operations such as addition (combines with, sum, increased by, plus, etc.), subtraction (difference of, minus, decreased by, etc.), multiplication (product of, times, of), and division (ratio of, quotient of). It also discusses the significance of understanding equal signs and arrows in chemical equations, which can indicate formation (form, produce, create, yield, result). The paragraph then delves into the specifics of writing balanced chemical equations from words, highlighting the identification of states (aqueous, liquid, solid, gas), reactants (reacts with, combines with), and products (forms, yields, creates, etc.), as well as the types of chemical reactions (decomposition, single replacement, combustion, precipitation). An example is provided to illustrate how to translate a word problem into a balanced chemical equation, focusing on the keywords that indicate the state of matter, reactants, products, and type of reaction.
🧪 Utilizing 'Of' in Chemistry Word Problems
The second paragraph focuses on the use of the word 'of' in chemistry word problems, particularly in topics like dilutions and molarity. It explains that molarity (represented by M) is a measure of the concentration of a solution and that dilution problems involve calculating volumes (V) and molarities (M) using specific formulas. The paragraph emphasizes the importance of correctly identifying and labeling the given values in a problem as M1, V1, M2, and V2 to avoid errors. It provides an example of a dilution problem and shows how to use the word 'of' to associate the initial molarity and volume, and how to determine which molarity is M2 and which volume is V2. The paragraph also touches on solution stoichiometry and the role of 'of' in identifying the correct volumes and molarities for different solutions. It concludes with encouragement to practice these skills and directs viewers to additional practice problems on the speaker's website.
Mindmap
Keywords
💡Word Problems
💡Addition
💡Subtraction
💡Multiplication
💡Division
💡Balanced Chemical Equation
💡States of Matter
💡Reactants and Products
💡Chemical Reaction Types
💡Dilution
💡Molarity
💡Solution Stoichiometry
Highlights
Chemistry word problems can be translated using common words and phrases that relate to mathematical operations.
Keywords for addition include 'combines with', 'the sum', 'increased by', 'plus', 'the total of', and 'and'.
Subtraction is indicated by terms like 'the difference of', 'minus', 'decreased by', 'less than', and 'reduced by'.
Multiplication is suggested by 'the product of', 'times', or simply the word 'of'.
Division is often denoted by 'the ratio of' or 'the quotient of'.
Words like 'form', 'produce', 'create', 'yield', and 'result' refer to an equal sign or an arrow in a chemical equation.
Chemical equations have four different states: aqueous, liquid, solid, and gas.
Aqueous state is indicated by terms like 'dissolved', 'dissociated', 'ionized', or 'aqueous'.
Solids are often represented by keywords such as 'precipitate' or 'metal'.
Gases are identified by words like 'vapor', 'gaseous', or references to diatomic molecules.
Reactants in a chemical equation are marked by 'reacts with' or 'combines with', and are placed on the left side of the arrow.
Products are signified by 'forms', 'yields', 'creates', 'results in', 'converts into', or 'produces', and are on the right side of the arrow.
Types of chemical reactions are hinted by keywords like 'decomposes' for decomposition, 'rust' for single replacement, 'combusts' or 'burns' for combustion, and 'precipitate' for precipitation reactions.
Balancing chemical equations involves identifying reactants, products, and the type of reaction.
The word 'of' in chemistry problems often signifies multiplication and can help in setting up dilution and molarity problems.
Dilution problems involve volumes and molarities, using formulas that relate initial and final molarities and volumes.
Molarity questions, especially in solution stoichiometry, require understanding the relationship between volume and solution concentration.
Properly identifying and labeling values in word problems is crucial for accurate problem-solving.
Practice problems are available on melissa.help/practice to master the translation of chemistry word problems.
Transcripts
Browse More Related Video

4.2 Types of Chemical Reactions | High School Chemistry

Predicting The Products of Chemical Reactions - Chemistry Examples and Practice Problems
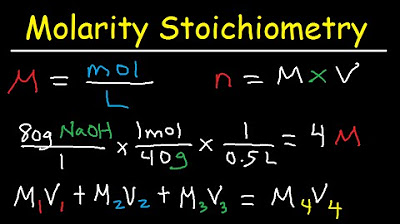
Molarity Dilution Problems Solution Stoichiometry Grams, Moles, Liters Volume Calculations Chemistry

How To Balance Chemical Equations

Types of Chemical Reactions

Chemical Reactions - Combination, Decomposition, Combustion, Single & Double Displacement Chemistry
5.0 / 5 (0 votes)
Thanks for rating: